Abstract
Background. The first Nordic protocol for three-dimensional (3D) planned radiotherapy in locally advanced cervical cancer was the prospective NOCECA study (1994–2000). NOCECA consisted of computed tomography (CT)-based 3D conformal external beam radiotherapy (EBRT) with a simultaneous integrated boost (SIB) to the primary tumour combined with brachytherapy (BT) based on x-ray imaging. In NOCECA the planning aim was to achieve 80 Gy at point A from EBRT and BT combined. However, the balance of dose between EBRT and BT was determined by tumour size at diagnosis with more EBRT dose given to point A and less by BT in more advanced stages. In 2005 image-guided adaptive brachytherapy (IGABT) based on magnetic resonance imaging (MRI) and optimisation of the BT dose distribution to the remaining tumour and cervix at time of BT (HR CTV) was introduced in Aarhus. EBRT remained like in NOCECA until 2008 when the SIB to the primary tumour was abandoned and IMRT was introduced as routine technique. In this study, we report outcome of our first five-year experience with IGABT using our NOCECA cohort as reference. Material and methods. The NOCECA cohort comprising 99 patients was compared with 140 consecutive patients treated by IGABT. Patients with para-aortic nodes were excluded in NOCECA but were present in 9% of the patients treated with IGABT. No patient in NOCECA received chemotherapy whereas concomitant cisplatin was given to 79% of the IGABT patients. Results. With IGABT actuarial local control was 91% at three years. When comparing NOCECA with IGABT overall survival was significantly improved from 63% to 79% (p = 0.005). In parallel, both moderate and severe late morbidity were reduced by about 50% (p = 0.02). Conclusion. Introduction of IGABT reduced morbidity and generated a very high rate of local control, which likely has improved survival by at least as much as concomitant chemotherapy.
The treatment of locally advanced cervical cancer has for more than half a century been composed of pelvic external beam radiotherapy (EBRT) followed by intracavitary brachytherapy (BT), most often prescribed at point A [Citation1–5]. Realising the importance of local control [Citation6,Citation7] and the limited capabilities of standard point A-based BT in extensive disease, a Nordic protocol for computed tomography (CT)-based EBRT in locally advanced cervical cancer (NOCECA) was initiated in 1994. NOCECA was quite advanced for its time including three-dimensional (3D) target contouring and treatment planning. NOCECA also employed a simultaneous integrated boost (SIB) to the primary tumour and uterus. The planning aim in NOCECA was to reach a cumulative physical dose by EBRT and BT of 80 Gy to point A within a short overall treatment time (6–7 weeks). Intracavitary BT was used based on standard loading and imaging of the implant was limited to orthogonal x-rays. In NOCECA radiotherapy was changed towards more EBRT and less BT dose to point A in tumours ≥ 8 cm at diagnosis.
In the period from 1994 to 2000 NOCECA accrued 631 patients from 10 Nordic centres including 99 patients from Aarhus. Apart from an abstract NOCECA was never published, even though survival in the NOCECA study was comparable to the experimental arms in similar American studies with concomitant chemotherapy [Citation8–10]. NOCECA has perhaps for this reason strongly influenced the Nordic approach to the treatment of locally advanced cervical cancer and the basic concept is still used in the Nordic countries, but nowadays together with concomitant chemotherapy.
Since the times of NOCECA it has been realised that even large cervix tumours may regress significantly during the first 2–3 weeks of radiochemotherapy whereas others have poor response [Citation11,Citation12]. In addition, magnetic resonance imaging (MRI) including functional sequences performed before and during radiotherapy has shown potential for providing important predictive information [Citation13,Citation14]. This adds time as a fourth dimension to the possibilities for individualised adaptation of BT dose delivery. In fact, locally advanced cervical cancer has been shown to be a prime candidate and model example for 4D adaptive radiotherapy [Citation15]. The major breakthrough came in 2005 with publication of the 4D target concept for image-guided adaptive brachytherapy (IGABT) by GEC ESTRO, addressing the residual GTV, the cervix and remaining tumour at time of BT defined as the high risk clinical target volume (HR CTV). The tumour volume at diagnosis was also taken into account and included in the so called intermediate risk volume (IR CTV). Functional MRI has been able to identify distinct tissue characteristics for the GEC ESTRO target structures [Citation16] supporting the concept and the idea of delivering specific doses to different volumes according to risk of remaining tumour cells at time of BT [Citation17,Citation18]. Several studies have shown that IGABT is able to selectively deliver very high doses (> 90 Gy) to the HR CTV leading to an improved therapeutic ratio [Citation19–25].
In late 2005 MRI-based IGABT was introduced in Aarhus. Initially the 3D conformal EBRT component from NOCECA was combined with MRI-guided 4D intracavitary BT [Citation26]. Later combined intracavitary/interstial BT was introduced and the SIB delivered by EBRT was abandoned, resulting in an almost even balance of doses delivered from EBRT and BT to the HR CTV [Citation12,Citation27]. The aim of the present paper is to analyse the implementation of IGABT and to quantify the clinical outcome in the first 140 patients treated 2005–2011 in a Nordic perspective with the Aarhus contribution to NOCECA as reference.
Material and methods
Patients and staging
This analysis includes 99 patients accrued from February 1994 to March 2000 to the NOCECA study and 140 consecutive patients treated with IGABT from November 2005 to February 2011. Five patients were not included in the IGABT cohort: three patients with uncertainty concerning the origin of the tumour (transitio-cellular or clear cell carcinomas) and two patients where distant metastases were diagnosed during treatment.
In both cohorts FIGO staging was based on gynaecological examination in general anaesthesia performed jointly by gynaecologist and oncologist [Citation28]. NOCECA included patients with stage IIB–IVA disease, but excluded patients with pathologically enlarged para-aortic nodes on CT. In the IGABT cohort the patients were additionally investigated using MRI of the pelvis and FDG positron emission tomography (PET)-CT. The IGABT cohort also included patients with stage I disease referred for primary radiochemotherapy because of age, medical inoperability or positive nodes. In addition, patients with PET positive para-aortic nodes and patients treated with neoadjuvant chemotherapy due to extensive loco-regional disease at presentation were part of the IGABT cohort. Pathological verification of squamous cell carcinoma, adenocarcinoma or adeno-squamous cell cancer was obtained in all patients in both cohorts.
Treatments used in NOCECA
The patients included in NOCECA were treated according to tumour size at diagnosis. Type 1 was used in tumours < 8 cm (primarily stage IIB) and consisted of 45 Gy/25 fx whole pelvic 3D conformal EBRT to the L4–L5 interspace (CTV-P) using AP-PA or four-field box technique with a SIB to the primary tumour and uterus (CTV-U) reaching 50 Gy/25 fx by lateral opposed fields. The doses were specified to the ICRU reference point in the central pelvis. In addition 3 fractions of 10 Gy to point A using intracavitary medium dose rate (MDR) or pulsed dose rate (PDR) BT was given with a maximal overall treatment time of six weeks including BT. Type 2 was used for tumours ≥ 8 cm (mainly stage IIIB). CTV-P was treated with 45 Gy/25 fx and CTV-U with 50 Gy/25 fx. In addition a subsequent boost of 10 Gy/5 fx were delivered to the CTV-U using the lateral opposed fields. BT consisted of 2 fractions of 10 Gy to point A, delivered within a maximal OTT of seven weeks. Type 3 was used in patients not suitable for intracavitary BT giving 50.4 Gy/28 fx to CTV-P and 56 Gy/28 fx to CTV-U plus a succeeding boost of 24 Gy/12 fx using the (if possible reduced) lateral opposed fields. The protocol did not specify a dose or technique for boosting of pathological pelvic nodes for any of the NOCECA types of EBRT. In Aarhus 60 Gy was usually delivered in 2 Gy fractions as a SIB given either via the lateral fields used for the CTV-U or by separate AP-PA portals.
BT based on x-ray imaging was originally delivered by MDR caesium tube-and box system with a dose rate of about 1 Gy/h at point A. PDR afterloading with plastic tandem-ring intracavitary (IC) applicators (GammaMed, Varian) was introduced in 1996 closely imitating the same average dose rate and point A-based pear-shaped dose distribution (standard plan) as obtained with the caesium tube and box system [Citation26,Citation27,Citation29]. For each PDR fraction 10 hourly pulses of 1 Gy to point A were delivered.
Treatments used for the IGABT cohort
Originally the NOCECA type 1 or type 2 combinations of EBRT and BT were used for IGABT. However, from 2008 IMRT and later VMAT were gradually replacing 3D conformal EBRT and the SIB to the tumour and uterus (CTV-U) was abandoned (). Instead patients with no pathological nodes received 45 Gy/25 fx to the whole pelvis (CTV-E) and patients with pathological nodes received 50 Gy/30 fx to CTV-E and a SIB of 60 Gy/30 fx to all pathological node(s) (CTV-N). A volume was generated around the GTV at the cervix with a 1 cm isotropic margin to ensure a homogenous dose in the central pelvis where BT was later applied. The para-aortic region to the level of L1-L2 was included in CTV-E if pathological nodes were present at the common iliac region or higher. In these cases the para-aortic part of CTV-E was treated with 45 Gy/30 fx.
Table II. Treatment characteristics for the IGABT and NOCEAC cohorts of patients with locally advanced cervical cancer treated at Aarhus University Hospital.
BT was delivered with the same PDR afterloader and basic tandem-ring plastic IC applicator as used in the NOCECA group, but the applicator was supplemented with a needle cap for titanium or plastic needles when a combined intracavitary-interstitial (IC/IS) was neccesary [Citation12,Citation26,Citation30,Citation31]. Concurrently the implant strategy was changed using the first implant (BT0) only for the purpose of preplanning of the subsequent two implants (BT1 and BT2) where the BT doses was then delivered in 15–20 hourly pulses [Citation12]. MRI with the applicator in situ was performed for each implant. T1 sequences were used for applicator reconstruction. Contouring of targets and organs at risk as well as dose optimisation was performed on T2-weighted sequences. The combined dose of EBRT and BT was calculated using simple DVH parameter addition [Citation32] and the concept of equivalent dose in 2 Gy fractions (EQD2) of the linear quadratic model assuming α/β = 10 for tumour, α/β = 3 for organs at risk and a repair halftime = 1.5 h [Citation17]. BT dose prescription was from the beginning based on the GEC ESTRO target concept [Citation17,Citation18] with a planning aim of reaching a D90 of at least 85 Gy (EQD2) to HR CTV and 60 Gy (EQD2) to IR CTV [Citation27]. For organs at risk the DVH constraints were 90 Gy (EQD2) for D2 cm3 of bladder and 75 Gy (EQD2) to D2 cm3 of rectum and sigmoid. In 2009 the constraint for rectum and sigmoid was lowered to 70 Gy (EQD2).
Weekly concomitant cisplatin 40 mg/m2 was given to all patients with sufficient kidney and bone marrow function. Neoadjuvant chemotherapy employing 2–4 courses of cisplatin, ifosfamide and 5FU were given in case the loco-regional tumour burden was found to be too extensive for initial definitive radiochemotherapy [Citation33].
Follow-up and statistical analysis
In NOCECA the patients were followed prospectively with gynaecological examination every three months the first year, every six months in year 2–3 and every 12 months in year 4–5. Imaging was not routinely performed and MRI and PET-CT not generally available. Morbidity was scored by use of the RTOG scale [Citation34]. Patients treated with IGABT were followed using of the same follow-up intervals. All patients were routinely scanned with MRI and PET-CT at three month follow-up and again with MRI at 12 months follow-up. MRI and PET-CT was also performed in case a recurrence was suspected. Morbidity was scored prospectively either using the RTOG scale or by the CTC v 3.0 for patients included in the Embrace study [Citation35,Citation36]. For the present paper the CTC scores were retrospectively evaluated and assigned a corresponding score in the RTOG system as close as possible. The present paper is based on data analysis performed in 2000 for NOCECA [Citation29] and in 2012 for IGABT [Citation37] both having a median follow-up time of about three years.
The patient and tumour characteristics for the NOCECA and IGABT groups were compared using a χ2-test or t-test. Actuarial estimates of disease control, survival and morbidity were calculated using Kaplan-Meier statistics. For the actuarial analysis of tumour control, patients without recurrence were censored at last follow-up or date of death. All patients with recurrent disease at time of death were considered dead from cervical cancer. Actuarial morbidity was analysed as morbidity grade 1 or worse, grade 2 or worse, and grade 3 or worse. Patients were censored from the analysis of morbidity in case of a recurrence or death. The log-rank test was used to test the equivalence of estimates of survival and complications for NOCECA and IGABT. A probability < 0.05 (two-sided) was considered to indicate significance. Correction for mass significance was not performed.
Results
Stage distribution, tumour size and lymph node involvement were different in the NOCECA and IGABT groups (). FIGO stage IIB was the most common stage in both groups, but stage IB and IVA were more common in the IGABT group and stage IIIB more common in the NOCECA group. Clinical tumour size at diagnosis was larger for the NOCECA group by about 1 cm. Pelvic nodal involvement was not recorded in NOCECA, but in the IGABT group pathological nodes on imaging were found in the pelvis in 41%, and in the para-aortic region in 9% of the patients. No significant difference was found in the distribution of histological subtypes.
Table I. Patient and tumour characteristics for two cohorts treated with either MRI-guided IGABT or x-ray-based BT (NOCECA) at Aarhus University Hospital for locally advanced cervical cancer.
Para-aortic EBRT was administered in 13% by IMRT in the IGABT group and altogether IMRT was used in 39% in this group (). The NOCECA EBRT techniques type 1 and 2 were used in 38% of the IGABT patients. The dose delivered by EBRT to the central pelvis was nonetheless 7 Gy less for the IGABT group compared to the NOCECA cohort (p < 0.001). In the NOCECA group about half of the BT was delivered with caesium and half with PDR afterloading. Ten patients were treated with EBRT only because BT was not possible. All patients in the IGABT group were treated with BT. In 98% of the cases contouring and treatment planning of BT was based on MRI. Combined IC/IS technique was used in 43% of the IGABT patients. Chemotherapy was only administered to the IGABT patients. Concomitant cisplatin was given to 78% and neoadjuvant chemotherapy to 7% of the IGABT patients. Treatment was completed in all patients in the IGABT group. Two patients did not complete treatment in the NOCECA group, one because of lethal bleeding and one because of deteriorating general condition. Overall treatment time (including BT, but excluding neoadjuvant chemotherapy) was significantly shorter with NOCECA than with IGABT. However, overall treatment time ≤ 55 days was realised for 92% and 94% of the patients for IGABT and NOCECA, respectively.
Evidently there are no DVH parameters available for the cumulative dose of EBRT and BT in the NOCECA cohort. However, in the impact of optimisation of a standard point A-based BT plan as used in NOCECA is demonstrated in the first 70 patients of the IGABT group treated 2005–2008. With a standard BT dose distribution the planning aims for targets and OARs were fulfilled in 15 patients (27%). The effect of BT optimisation is demonstrated in . In the first 70 patients we were able to fulfil the planning aim and respect the DVH constraint in 56 patients (80%). In parallel with the more widespread use of the IC/IS BT technique (increasing from 39% to 47%) the dose distribution and DVH parameters were further improved in the 70 patients treated 2009–2010, with 65 (93%) patients achieving both the planning aim (HR CTV D90 > 85 Gy) and respecting the OAR constraints (D2 cm3 bladder < 90 Gy, D2 cm3 rectum & sigmoid < 75 Gy). The DVH parameters of the IGABT group in terms of EQD2 of the cumulative EBRT and BT dose are given in . The ICRU rectal point dose and the D2 cm3 for bladder, rectum and sigmoid were all significantly lowered by 2–6 Gy when comparing the first and the last 70 consecutive patients of the IGABT group. There was no change in dose to the ICRU bladder point and D90 of HR CTV and only a minor decrease in the D90 of IR CTV.
Figure 1. Cumulative dose (EQD2) of EBRT and IGABT in terms of D2 cm3 to the sigmoid plotted as a function of the D90 to the HR CTV. DVH parameters for a standard point A-based plan for the first 70 consecutive patients (2005–2008) is shown in panel A. Optimised DVH parameters actually used for treatment in all 140 patients are given in panel B divided into the first 70 patients treated 2005–2008 (grey circles) and the last 70 consecutive patients treated 2009–2011 (white circles). The planning aim of 85 Gy to D90 of HR CTV and the DVH constraints of first 75 and later 70 Gy are indicated by dotted lines.
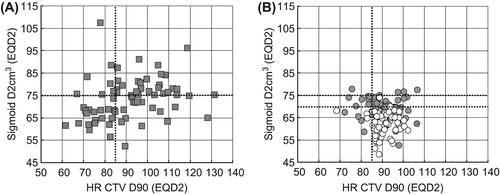
Table III. DVH parameters for EBRT and BT calculated in EQD2 (mean, range) for 140 consecutive patients treated with IGABT in the period 2005–2011 at Aarhus University Hospital. The DVH parameters for the 70 first and 70 last consecutively treated patients are given in separate columns. In addition, standard point A-based DVH parameters are given for the first 70 patients treated 2005–2008. Number of patients fulfilling the planning aim (HR CTV D90 > 85 Gy, D2 cm3 bladder < 90 Gy and D2 cm3 rectum and sigmoid < 75 Gy) are also indicated.
With IGABT we observed five patients with incomplete local remission and six patients with local recurrence, i.e. within the HR CTV and/or IR CTV volume. In the D90 to the HR CTV is shown as a function of HR CTV volume. The HR CTV volume was significantly larger and the D90 of HR CTV significantly lover (p = 0.002) in the patients with local failure. Thus, HR CTV D90 was less than 90 Gy in all local failures and only two patients with a local failure had a HR CTV volume smaller than 30 cm3. The actuarial local control at three years for the IGABT patients was 91% (, ). We have no information on the local control rate for NOCECA, but actuarial pelvic control (in-field) was 76% at three years for NOCECA and 85% in the IGABT group (p = 0.12). With IGABT both overall- and cancer-specific survival were significantly improved (p < 0.005) by 16% and 19% at three years, respectively. Overall survival was still significantly improved when only stage IIB–IV patients were compared (p = 0.01). Both moderate gastrointestinal and vaginal morbidity were significantly reduced with IGABT (p < 0.001). There were few patients with severe morbidity, but overall G3 morbidity was reduced by more than 50% with IGABT (p = 0.02). The actuarial curves for combined grade 3 urological and gastrointestinal morbidity are shown in demonstrating a significant decrease from 10% to 3% (p = 0.01).
Figure 2. D90 of HR CTV shown as a function of HR CTV volume in 140 consecutive patients treated with MRI-based IGABT for locally advanced cervical cancer. Local failure was observed in 11 patients (black spot). The planning aim for D90 of HR CTV and the median D90 and volume of HR CTV are indicated by dotted lines.
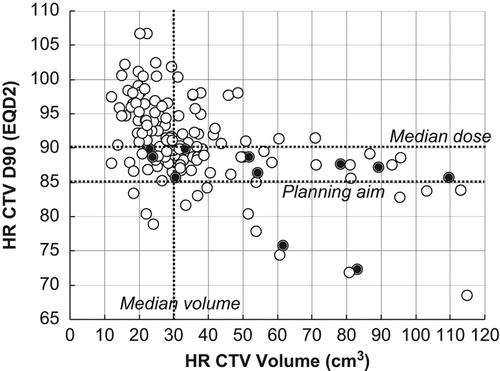
Figure 3. Actuarial local control, overall survival and ≥ grade 3 combined urological-gastrointestinal morbidity in 140 patients treated with IGABT (black lines). For comparison the curves for overall survival and morbidity in 99 patients treated with 2D x-ray-based brachytherapy (NOCECA) are indicated (grey lines). Patient number at risk for overall survival is indicated below the x-axis.
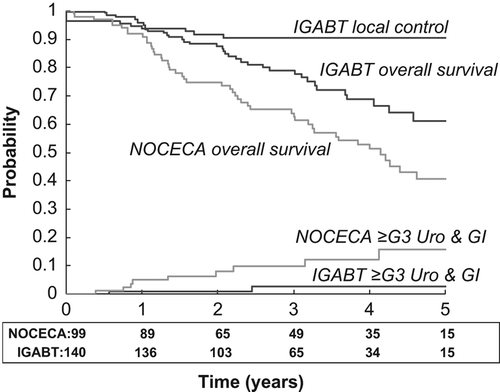
Table IV. Outcome in terms of actuarial three-year disease control, survival and morbidity in 140 consecutive patients treated with MRI-guided IGABT compared with 99 patients treated with x-ray-based BT (NOCECA).
Discussion
Based on a comparison of two prospectively followed cohorts of patients, this study shows an absolute improvement in survival by approximately 15% and a relative reduction of moderate and severe morbidity by about 50% in women treated with radiochemotherapy including MRI-based IGABT and partly also IMRT. Evidently inter-era comparisons are prone to several sources of bias and therefore difficult to interpret with regard to cause-effect relationships. However, based on the literature and circumstantial evidence it is likely that the introduction of IGABT has had a significant effect beyond that of concomitant chemotherapy, IMRT and any imbalance in prognostic factors.
The FIGO stage distribution in the current IGABT cohort is similar to published and ongoing studies with MRI-guided BT in cervix cancer [Citation19,Citation21,Citation22,Citation38], reflecting that all consecutive patients treated with definitive radiochemotherapy were included in the IGABT cohort. In contrast, the NOCECA cohort was systematically selecting stage IIB–IVA patients and excluded patients with paraaortic nodes, explaining the difference in stage distribution between the two cohorts. Other factors include the difference in diagnostic work up, and although a diagnostic CT scan was used in the NOCECA patients, it is possible that some NOCECA patients would have been referred for palliative treatment on the basis of a positive PET-CT had it been available. While FIGO staging in principal is purely clinical [Citation28,Citation39], the systematic use of MRI may have influenced the clinical staging in the IGABT cohort as MRI have been shown to be significantly better in ruling out parametrial invasion and advanced disease than clinical examination [Citation40,Citation41]. Most likely FIGO staging without MRI support is somewhat broader for allocation to stage IIB and beyond as evidenced, i.e. by a very high concordance rate between clinical and pathological stage I in operated patients found among surgically treated patients from the NOCECA era [Citation42]. This may also explain the relative high number of stage IIIB cases in NOCECA as compared to contemporary cohorts of locally advanced cervical cancer including MRI in the diagnostic work-up [Citation19,Citation20,Citation38]. Multivariate and subgroup analysis was not performed as the number of patients was relatively small and detailed knowledge about many of the possible confounding covariates was not available for the NOCECA cohort. However, as a whole it is reasonable to assume that there is no major imbalance of poor prognostic factors in one of the cohorts which is also demonstrated by the captured survival advantage observed when only stage IIB–IV patients are compared. As morbidity in both time periods were assessed prospectively and analysed by actuarial methods it is unlikely that the dramatic decrease in late morbidity is due to bias in patient selection. The median follow-up time was also similar between the two groups.
The use of chemotherapy in the IGABT patients may explain the improved local control and survival. However, based on the most recent meta-analysis on the magnitude of the effect of concomitant chemotherapy an improved survival by about 6% can be achieved and the benefit is even smaller in stage III–IV disease [Citation43]. A population-based period analysis from Germany points to a similar figure for the effect of concomitant chemotherapy [Citation44]. Neoadjuvant chemotherapy was used in 10 patients with very advanced disease in the IGABT cohort to decrease the tumour burden before curative radiotherapy. Neoadjuvant chemotherapy has so far not been shown to increase survival in patients treated with definitive radiotherapy [Citation45]. It is therefore unlikely that the improved therapeutic ratio observed for IGABT is attributed to neoadjuvant chemotherapy. Recently concomitant chemotherapy has been shown to enhance the risk of late vaginal and skeletal morbidity [Citation46]. If anything, concomitant cisplatin would also be expected also increase the risk of urological and gastrointestinal morbidity. Compared to the present results the impact of switching from 2D x-ray-based BT to IGABT in terms of reducing morbidity may therefore be even greater in the setting of established radiochemotherapy [Citation21].
As expected the change in EBRT practice with abandoning of the SIB to the primary tumour in the latter part of the IGABT era led to a significant reduction of the EBRT dose contribution to the cervix and primary tumour in the central pelvis. The elective dose in the periphery of the pelvis was unchanged when comparing the IGABT patients and NOCECA patients (). As shown by Logsdon et al., the optimal ratio between tumour control and complications is achieved with a combination of 45–50 Gy of whole pelvic EBRT combined with BT [Citation47]. There are two probable explanations for this observation. First, an EBRT boost to the cervix significantly increases the volume of OAR irradiated to intermediate dose levels (> 60 Gy). The importance of intermediate dose levels for faecal incontinence after gynaecological radiotherapy has recently been shown [Citation48]. Second, an EBRT boost cannot produce the same high dose region in the middle of the GTV as BT [Citation49–51]. The change in EBRT technique from 3D conformal to IMRT has been shown to decrease morbidity significantly [Citation52]. It is difficult to asses the isolated impact of IMRT in the present study with only 40% of the IGABT patients being treated with IMRT. In addition, IMRT was specifically used for boosting of pathological nodes and for para-aortic elective nodal irradiation. However, we did observe a significant reduction in G2 gastrointestinal morbidity which to some extent could be attributed to IMRT.
Evidently there is no 3D BT dose information from NOCECA. However, illustrates the impact on the DVH parameters when 2D x-ray-based BT is replaced with individualised IGABT. We have previously shown that IGABT and the use of the GEC ESTRO target concept significantly improved the DVH parameters compared to point A-based BT [Citation26,Citation27]. This paper further demonstrates that the use of combined IC/IS BT applicators and BT preplanning [Citation12] has the potential for further improving DVH parameters in IGABT and likely plays an important role for reduction of side effects and improving local control.
The importance of the D90 of HR CTV for both local control and survival was first demonstrated by the Vienna group showing that an increase in the mean D90 of the HR CTV from 81 Gy to 90 Gy (EQD2) in large tumours significantly improved local control and increased overall survival by about 10% [Citation20,Citation53]. Emerging evidence from multicentre studies also points to a clear dose-response relationship [Citation38,Citation54]. Based on these observations and the improved capabilities for dose delivery by IC/IS BT [Citation12] the planning aim for the D90 of HR CTV has since 2012 been increased from 85 to 90 Gy. In the present study we obtained a mean D90 for HR CTV of 91 Gy (EQD2) resulting in a local control rate of 91%. For overall survival there was an improvement of about 15% between NOCECA and IGABT. Taking the estimated effect of concomitant chemotherapy into account we conclude that IGABT has at least a similar impact on overall survival. This balance of effect is however, most likely influenced by tumour size with more effect from IGABT and less from concomitant chemotherapy in large tumours and vice versa in small tumours [Citation20,Citation43].
The overall actuarial rate of severe late morbidity (15%) in the NOCECA patients reported here is similar to previous studies with x-ray-based BT [Citation55,Citation56]. We have previously found that late morbidity was similar for NOCECA patients treated with caesium tube and box and point A-based PDR afterloading [Citation29]. As demonstrated in the optimisation process involved in IGABT curbed the outliers of very high doses close to the BT applicator (D2 cm3) which may not be reflected in the traditional ICRU points used for x-ray-based BT [Citation51,Citation57]. In addition, we could in the vast majority of the patients respect DVH constraints for bladder, rectum and sigmoid previously used successfully for HDR brachytherapy [Citation19,Citation20] with similar low rates of G3 morbidity. We also observed a significant impact of IGABT on moderate and severe late effects in the vagina. With IGABT it is often possible to reduce the vaginal loading, especially in small tumours, thereby keeping the high dose volume out of the vagina. In addition, with the use of the IC/IS technique in large tumours the need for heavy vaginal loading is minimised.
Conclusion
The implementation of IGABT has profoundly improved the therapeutic ratio of radiochemotherapy in locally advanced cervical cancer yielding a very high rate of local control and a significant reduction of moderate and severe late effects. The improved rate of local control has likely improved overall survival by about 5–10%.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by CIRRO – The Lundbeck Foundation Center for Investigational Research in Radiation Oncology and the Danish Cancer Society.
References
- Perez CA, Grigsby PW, Chao KS, Mutch DG, Lockett MA. Tumor size, irradiation dose, and long-term outcome of carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys 1998;41:307–17.
- Horiot JC, Pigneux J, Pourquier H, Schraub S, Achille E, Keiling R, et al. Radiotherapy alone in carcinoma of the intact uterine cervix according to G. H. Fletcher guidelines: A French cooperative study of 1383 cases. Int J Radiat Oncol Biol Phys 1988;14:605–11.
- Viswanathan AN, Creutzberg CL, Craighead PS, McCormack M, Toita T, Narayan K, et al. International brachytherapy practice patterns: A survey of the gynecological cancer intergroup (GCIG). Int J Radiat Oncol Biol Phys 2012;82:250–5.
- Pötter R, van Limbergen E, Gerstner N, Wambersie A. Survey of the use of the ICRU 38 in recording and reporting cervical cancer brachytherapy. Radiother Oncol 2001;58: 11–8.
- Gerbaulet A, Pötter R, Mazeron JJ, Meertens H, van Limbergen E. The GEC ESTRO handbook of brachytherapy, 1st ed. Leuven, Belgium: ACCO; 2002.
- Eifel PJ. The importance of locoregional control of cervical cancer – why is it still controversial?Cancer J Sci Am 1996;2:253–5.
- Pedersen D, Bentzen SM, Overgaard J. Continuous or split-course combined external and intracavitary radiotherapy of locally advanced carcinoma of the uterine cervix. Acta Oncol 1994;33:547–55.
- Jakobsen A. External radiation of cervix cancer with a concomittant boost. Int J Gynecol Cancer 2004;14:11–2.
- Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. New Engl J Med 1999;340:1144–53.
- Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr. et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol 1999;17:1339–48.
- Dimopoulos JC, Schirl G, Baldinger A, Helbich TH, Potter R. MRI assessment of cervical cancer for adaptive radiotherapy. Strahlenther Onkol 2009;185:282–7.
- Fokdal L, Tanderup K, Hokland SB, Rohl L, Pedersen EM, Nielsen SK, et al. Clinical feasibility of combined intracavitary/interstitial brachytherapy in locally advanced cervical cancer employing MRI with a tandem/ring applicator in situ and virtual preplanning of the interstitial component. Radiother Oncol 2013;107:63–8.
- Mayr NA, Wang JZ, Lo SS, Zhang D, Grecula JC, Lu L, et al. Translating response during therapy into ultimate treatment outcome: A personalized 4-dimensional MRI tumor volumetric regression approach in cervical cancer. Int J Radiat Oncol Biol Phys 2010;76:719–27.
- Andersen EK, Kristensen GB, Lyng H, Malinen E. Pharmacokinetic analysis and k-means clustering of DCEMR images for radiotherapy outcome prediction of advanced cervical cancers. Acta Oncol 2011;50:859–65.
- Pötter R. Image-guided brachytherapy sets benchmarks in advanced radiotherapy. Radiother Oncol 2009;91:141–6.
- Haack S, Pedersen EM, Jespersen SN, Kallehauge JF, Lindegaard JC, Tanderup K. Apparent diffusion coefficients in GEC ESTRO target volumes for image guided adaptive brachytherapy of locally advanced cervical cancer. Acta Oncol 2010;49:978–83.
- Pötter R, Haie-Meder C, Limbergen EV, Barillot I, Brabandere MD, Dimopoulos J, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77.
- Haie-Meder C, Potter R, van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005;74:235–45.
- Pötter R, Dimopoulos J. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol 2011;100:116–23.
- Pötter R, Dimopoulos J, Georg P, Lang S, Waldhausl C, Wachter-Gerstner N, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol 2007;83:148–55.
- Charra-Brunaud C, Harter V, Delannes M, Haie-Meder C, Quetin P, Kerr C, et al. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: Results of the French STIC prospective study. Radiother Oncol 2012;103:305–13.
- Haie-Meder C, Chargari C, Rey A, Dumas I, Morice P, Magne N. MRI-based low dose-rate brachytherapy experience in locally advanced cervical cancer patients initially treated by concomitant chemoradiotherapy. Radiother Oncol 2010;96:161–5.
- Chargari C, Magne N, Dumas I, Messai T, Vicenzi L, Gillion N, et al. Physics contributions and clinical outcome with 3D-MRI-based pulsed-dose-rate intracavitary brachytherapy in cervical cancer patients. Int J Radiat Oncol Biol Phys 2009;74:133–9.
- Wanderas AD, Sundset M, Langdal I, Danielsen S, Frykholm G, Marthinsen AB. Adaptive brachytherapy of cervical cancer, comparison of conventional point A and CT based individual treatment planning. Acta Oncol 2012;51:345–54.
- Nomden CN, de Leeuw AA, Roesink JM, Tersteeg RJ, Moerland MA, Witteveen PO, et al. Clinical outcome and dosimetric parameters of chemo-radiation including MRI guided adaptive brachytherapy with tandem-ovoid applicators for cervical cancer patients: A single institution experience. Radiother Oncol 2013;107:69–74.
- Lindegaard JC, Tanderup K, Nielsen SK, Haack S, Gelineck J. MRI-guided 3D optimization significantly improves DVH parameters of pulsed dose rate brachytherapy in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 2008;71:756–64.
- Tanderup K, Nielsen SK, Nyvang G, Pedersen EP, Røhl L, Fokdal L, et al. From point A to the sculpted pear: MR image guidance significantly improves tumour dose and sparing of organs at risk in brachytherapy of cervical cancer. Radiother Oncol 2010;94:173–80.
- Benedet JL, Bender H, Jones H, III, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet 2000;70:209–62.
- Lindegaard JC, Havsteen H, Christensen JJ, Tanderup K, Jakobsen A. Treatment outcome following replacement of continuous MDR with remote afterloading PDR brachytherapy in the treatment of advanced cervical cancer. Radiother Oncol 2000;56:S102.
- Dimopoulos JC, Kirisits C, Petric P, Georg P, Lang S, Berger D, et al. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: Clinical feasibility and preliminary results. Int J Radiat Oncol Biol Phys 2006;66:83–90.
- Kirisits C, Lang S, Dimopoulos J, Berger D, Georg D, Potter R. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: Design, application, treatment planning, and dosimetric results. Int J Radiat Oncol Biol Phys 2006;65:624–30.
- Andersen ES, Noe KO, Sorensen TS, Nielsen SK, Fokdal L, Paludan M, et al. Simple DVH parameter addition as compared to deformable registration for bladder dose accumulation in cervix cancer brachytherapy. Radiother Oncol 2013;107:52–7.
- Cadron I, Jakobsen A, Vergote I. Report of an early stopped randomized trial comparing cisplatin vs. cisplatin/ifosfamide/ 5-fluorouracil in recurrent cervical cancer. Gynecol Obstet Invest 2005;59:126–9.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6.
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–81.
- Tanderup K, Lindegaard JC, Kirisits C, Nkiwane K, Fidarova E, Haie-Meder C, et al. Embrace update. Radiother Oncol 2011;99:S22–3.
- Lindegaard JC, Fokdal L, Nielsen SK, Røhl L, Pedersen EM, Petersen LK, et al. Outcome of image guided adaptive brachytherapy in locally advanced cervical cancer. Radiother Oncol 2012;103:S41–2.
- Lindegaard JC, Haie-Meder C, Brunaud C, van Limbergen E, Kirisits C, Pötter R. Cervix cancer: Image guided adaptive brachytherapy in the setting of definitive radio-chemotherapy – status of current multi-centre studies. Radiother Oncol 2011;99:S226–7.
- Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 2009; 105:103–4.
- Thomeer MG, Gerestein C, Spronk S, van Doorn HC, van der HE, Hunink MG. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: Systematic review and meta-analysis. Eur Radiol 2013;23:2005–18.
- Stenstedt K, Hellstrom AC, Fridsten S, Blomqvist L. Impact of MRI in the management and staging of cancer of the uterine cervix. Acta Oncol 2011;50:420–6.
- Hoyer M, Ljungstroem B, Nyland M, Jakobsen A. Radical hysterectomy in cervical carcinoma stage Ib. Eur J Gynaecol Oncol 1990;11:13–7.
- Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer. a systematic review and meta- analysis of individual patient data from 18 randomized trials. J Clin Oncol 2008;26:5802–12.
- Chen T, Jansen L, Gondos A, Emrich K, Holleczek B, Luttmann S, et al. Survival of cervical cancer patients in Germany in the early 21st century: A period analysis by age, histology, and stage. Acta Oncol 2012;51:915–21.
- Tattersall MHN. Concomitant and neoadjuvant chemotherapy in conjunction with radiotherapy in the management of locally advanced cervical cancer. J Natl Cancer Inst Monogr 1996;21:101–3.
- Gondi V, Bentzen SM, Sklenar KL, Dunn EF, Petereit DG, Tannehill SP, et al. Severe late toxicities following concomitant chemoradiotherapy compared to radiotherapy alone in cervical cancer: An inter-era analysis. Int J Radiat Oncol Biol Phys 2012;84:973–82.
- Logsdon MD, Eifel PJ. Figo IIIB squamous cell carcinoma of the cervix: An analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapy. Int J Radiat Oncol Biol Phys 1999; 43:763–75.
- Alevronta E, Lind H, Al-Abany M, Waldenstrom AC, Olsson C, Dunberger G, et al. Dose-response relationships for an atomized symptom of fecal incontinence after gynecological radiotherapy. Acta Oncol 2013;52:719–26.
- Assenholt MS, Petersen JB, Nielsen SK, Lindegaard JC, Tanderup K. A dose planning study on applicator guided stereotactic IMRT boost in combination with 3D MRI based brachytherapy in locally advanced cervical cancer. Acta Oncol 2008;47:1337–43.
- Georg D, Kirisits C, Hillbrand M, Dimopoulos J, Potter R. Image-guided radiotherapy for cervix cancer: High-tech external beam therapy versus high-tech brachytherapy. Int J Radiat Oncol Biol Phys 2008;71:1272–8.
- Tanderup K, Georg D, Pötter R, Kirisits C, Grau C, Lindegaard JC. Adaptive management of cervical cancer radiotherapy. Sem Radiat Oncol 2010;20:121–9.
- Kidd EA, Siegel BA, Dehdashti F, Rader JS, Mutic S, Mutch DG, et al. Clinical outcomes of definitive intensity-modulated radiation therapy with fluorodeoxyglucose- positron emission tomography simulation in patients with locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 2009;77:1085–91.
- Dimopoulos JC, Lang S, Kirisits C, Fidarova EF, Berger D, Georg P, et al. Dose-volume histogram parameters and local tumor control in magnetic resonance image-guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys 2009; 75:56–63.
- Tanderup K, Fokdal L, Sturdza AE, Mazeron R, Kirisits C, Lindegaard JC, et al. Dose-response for local control in image guided cervix brachytherapy in the retro EMBRACE study. Radiother Oncol 2013;106:S103–4.
- Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol 2004;22:872–80.
- Perez CA. Utererine cervix. In: Perez CA, Brady LW, editors. Principle and practice of radiation oncology. Philadelphia: Lippincott-Raven; 1997. pp 1733–834.
- Pötter R, Kirisits C, Fidarova EF, Dimopoulos JC, Berger D, Tanderup K, et al. Present status and future of high-precision image guided adaptive brachytherapy for cervix carcinoma. Acta Oncol 2008;47:1325–36.
