Abstract
To analyze clinical concepts, toxicity and treatment outcome in patients with brain and skull base tumors treated with photons and particle therapy. Material and methods. In total 260 patients with brain tumors and tumors of the skull base were treated at the Heidelberg Ion Therapy Center (HIT). Patients enrolled in and randomized within prospective clinical trials as well as bony or soft tissue tumors are not included in this analysis. Treatment was delivered as protons, carbon ions, or combinations of photons and a carbon ion boost. All patients are included in a tight follow-up program. The median follow-up time is 12 months (range 2–39 months). Results. Main histologies included meningioma (n = 107) for skull base lesions, pituitary adenomas (n = 14), low-grade gliomas (n = 51) as well as high-grade gliomas (n = 55) for brain tumors. In all patients treatment could be completed without any unexpected severe toxicities. No side effects > CTC Grade III were observed. To date, no severe late toxicities were observed, however, for endpoints such as secondary malignancies or neurocognitive side effects follow-up time still remains too short. Local recurrences were mainly seen in the group of high-grade gliomas or atypical meningiomas; for benign skull base meningiomas, to date, no recurrences were observed during follow-up. Conclusion. The specific benefit of particle therapy will potentially reduce the risk of secondary malignancies as well as improve neurocognitive outcome and quality of life (QOL); thus, longer follow-up will be necessary to confirm these endpoints. Indication-specific trials on meningiomas and gliomas are underway to elucidate the role of protons and carbon ions in these indications.
The treatment of brain and skull base tumors is challenging from various perspectives. On the one hand, some are characterized by aggressive histology, such as high-grade gliomas and meningiomas, while on the other hand, the close vicinity to organs at risk (OAR) especially in skull base lesions requires high-end treatment planning and delivery to optimally spare these radiation sensitive structures. To optimize this therapeutic window, individual choice of photons, protons and carbon ions can help increase dose and spare normal tissue.
Technical development in radiation oncology has continuously improved dose delivery to defined target volumes: For tumors such as gliomas in the primary treatment situation, three-dimensional (3D)-conformal radiotherapy delivers acceptable treatment plans and allows for safe and effective treatment, alone, or in combination with systemic treatment; stereotactic radiotherapy, either as single-dose radiosurgery (SRS) or within fractionated regimens produces highly conformal dose plans, and offers excellent options for low-grade tumors such as benign meningiomas, acoustic neuromas, or low-grade gliomas [Citation1–6]. Moreover, these techniques have enabled the radiation oncologist to perform re-irradiations with convincing efficacy, while with older techniques indication for re-irradiation had to be weighted diligently against potential side effects [Citation7–9]. For complex-shaped tumors, and tumors in the skull base region in close vicinity to OAR, intensity-modulated radiotherapy (IMRT) leads to improved dose distributions [Citation1].
Particle therapy is unique due to the physical properties of the ion beams. The inverted dose profile results in low-dose deposition in the entry channel of the beam, and a steep dose deposition in the tumor region, followed by a sharp dose-falloff. Especially regions of low and intermediate doses can be significantly reduced leading to an overall reduction of integral dose. Especially in benign lesions, the hypothesis is that the risk for side effects, predominantly long-term effects such as neurocognitive sequelae, secondary malignancies can be diminished as shown by several studies and calculations [Citation10–13]. However, to date, no prospective randomized data have shown this clinical improvement. High-LET particle beams, such as carbon ions, are associated with an increased relative biological effectiveness (RBE); for a number of indications, a benefit of carbon ions has been shown, however, again, no randomized studies exist to date compared to modern photon or proton treatment. Especially for glioblastomas, which are highly treatment resistant, this increase in RBE has been shown in the preclinical setting, and initial Phase I/II data from Japan showed convincing results [Citation14].
Since 2009, treatment of patients with brain tumors and skull base lesions has been performed at the Heidelberg Ion Therapy Center (HIT). The aim of the present study was to analyze clinical concepts, toxicity and treatment outcome in patients with brain and skull base tumors treated with particle therapy using the active raster scanning technology.
Patients and methods
Between November 2009 and February 2013, 260 patients with brain tumors and tumors of the skull base were treated at the HIT. Patients enrolled in and randomized within prospective clinical trials as well as cartitageous, bony or soft tissue tumors are not included into this analysis. Main histologies included meningioma (n = 107) for skull base lesions, pituitary adenomas (n = 14), low-grade gliomas (n = 51) as well as high-grade gliomas (n = 55) for brain tumors (). All patients were seen by a specialized clinical team and interdisciplinary discussion decided upon the indication for treatment.
Table I. Distribution of histologies/tumor entities treated with particle therapy.
A total of 176 patients were treated with protons (67%), 84 patients with carbon ions (33%); of the latter, 36 patients (43%) with photon radiotherapy and a carbon ion boost. One hundred and eighty-six patients were treated as primary radiotherapy (72%), and 74 with a second course of radiotherapy (28%). Median age was 48 years (range 1–85 years). Thirty patients were children younger than 18 years of age; of these, five were treated with anesthesia.
For each patient individually manufactured head masks were used with Scotch Cast™ or thermoplast masks as described previously [Citation15,Citation16]. For treatment planning the system by Siemens Oncology Care Systems (Syngo PT Planning, Siemens, Germany) was used, and target delineation was performed using the Siemens Oncologist Software (Siemens, Germany). For planning, a CT without and with contrast enhancement was acquired. A recent contrast- enhanced MRI was used for target volume delineation, additionally molecular imaging based on PET was used depending on the indication. For meningiomas, target definition enhanced by 68Ga-DOTATOC-PET and 18FET-PET, for gliomas, 18FET-PET was added for planning. All imaging was co-registered with the planning CT for target delineation.
We defined all OAR, as well as the tumor depending on tumor site and histology according to the ICRU criteria: The gross tumor volume (GTV) for any macroscopic tumor, the clinical target volume (CTV) for any microscopic spread depending on histology, and a planning target volume (PTV) for setup deviations which was defined depending on the location and the fixation device used.
Treatment planning based on the local effect model (LEM) was used as published in detail previously [Citation17–20]. For carbon ions, the optimization is based on this radiobiological model, which takes into account the variations of RBE within the radiation field and as a function of tissue type and fraction dose. This model allows the inclusion of organ and tumor specific RBE values. We adhered to the constant α/β value of 2 for intracranial lesions. Generally for protons, an RBE of 1.1 is used.
Patient positioning was evaluation prior to each fraction using orthogonal x-ray imaging position correction was performed using re-positioning of the treatment couch as well as using the pitch-and-roll feature of the robotic table system in some patients.
The median follow-up time is 12 months (range 2–39 months). All patients are seen for regular follow-up initially 4–6 weeks after completion of treatment, thereafter in three months intervals for the first year, or as needed clinically. Usually after the first year follow-up intervals are extended depending on the histology and the overall performance status of the patient. Follow-up examinations include a thorough clinical and neurological assessment, as well as contrast-enhanced MRI. Additional examinations are scheduled as needed clinically.
Follow-up assessment included thorough analysis of toxicities according to the Common Terminology Criteria CTCAE Version 4.1. Treatment response on imaging was based on the RECIST criteria.
Results
In all patients treatment could be completed without any unexpected severe toxicities; mild acute side effects included alopecia, fatigue, headaches as well as conjunctivitis and skin erythema. No side effects > CTC Grade III were observed. (middle column) summarizes clinical symptoms, patients’ complaints and symptoms reported within the first months after treatment.
Table II. Patients’ complaints recorded within the first 6 months of particle radiotherapy (middle column). Of 260, 157 patients were followed more than 6 months (right column).
Of all patients, 157 (60%) were followed for more than six months. Thus late toxicity was scored in these patients. To date, no severe late toxicities were observed, however, for endpoints such as secondary malignancies or neurocognitive side effects follow-up time still remains too short. In , patients’ complaints reported after six months of follow-up are documented.
Local recurrences were mainly seen in the group of high-grade gliomas or atypical meningiomas; for benign skull base meningiomas, to date, no recurrences were observed during follow-up.
High-grade gliomas
For WHO Grade III gliomas and glioblastomas treated for primary diagnosis, treatment consisted of 50 Gy E photons and a carbon ion boost of 18 Gy E, with concomitant and adjuvant temozolomide, according to the CLEOPATRA protocol. Of 34 patients, 12 patients developed a recurrence during follow-up with a median progression free survival time of 5.8 months. For re-irradiation (n = 21 patients), carbon ion radiotherapy alone was applied according to the CINDERELLA protocol; during follow-up, 13 patients developed tumor recurrences.
Meningiomas
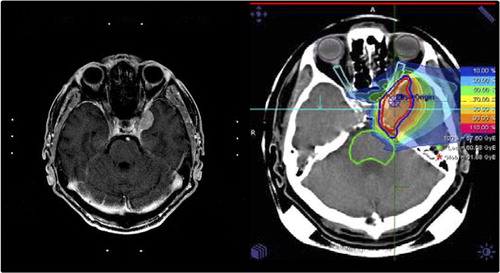
After primary radiotherapy, for low-grade meningiomas treated with proton radiotherapy with a median dose of 57.6 Gy E, local control over the follow-up time was 100%. shows a typical treatment plan of a skull base benign meningioma treated with protons, as well as a significant treatment response during follow-up. No recurrences were observed, and patients remained alive until the last included follow-up visit.
For high grade meningiomas, 17 of 36 patients developed a tumor recurrence after primary radiotherapy consisting of photon radiotherapy and a carbon ion boost. Local control was 54% at one year and 33% at two years.
Other histologies
For benign histologies such as craniopharyngioma or pituitary adenomas, local control was 100% after primary radiotherapy, delivered at a dose of 50.4–54 Gy E, depending on size, volume, shape or pre-existing symptoms of the patients.
Discussion
Clinical results after proton and carbon ion radiotherapy for primary brain tumors and certain tumors of the skull base region show promising outcome with very low side effects. Compared to photons, acute side effects such as hair loss or fatigue seem to be reduced, however, not completely absent. Combination with chemotherapy as performed in high-grade gliomas is well tolerated without any complications. Clinical workflow, especially with combined photon and particle boost treatment, require strict organization and streamlined treatment planning.
To date, clinical evidence of the superiority of particle beams is scarce. The optimized dose distributions with significant reduction of integral dose suggest a clinical benefit, however, long-term sequelae such as neurocognitive side effects of even rates of secondary malignancies in the adult population are difficult to assess and require very long-term standardized follow-up.
The indication for particle therapy may be set for two reasons, one being reduction of unwanted effects, the other a potential use of dose escalation due to the beneficial dose distributions, or the use of the higher RBE of carbon beams for certain high-grade histologies.
For gliomas, few data are available on particle therapy. For protons, dose escalation studies performed earlier and published by Fitzek and colleagues demonstrated a clear dose-response relationship for high-grade gliomas with patients treated with higher doses showing significantly increased survival, however, the rate of severe treatment-related toxicity, mainly symptomatic necrosis was relatively high. However, patients with necrosis showed a significantly increased overall survival, stressing the dose-response relationship of gliomas [Citation21]. With carbon ion radiotherapy, at NIRS, patients with glioblastomas and WHO Grade III astrocytomas were treated within a prospective trial with dose escalation; higher dose was significantly associated with increased outcome, and median survival of 17 months for glioblastoma is promising. To further elucidate the role of carbon ion radiotherapy, studies on primary and recurrent glioblastomas are currently recruiting patients at the HIT [Citation22,Citation23]; for primary glioblastomas, combination with temozolomide according to the present treatment standard is applied. Early data has shown promising responses and low rates of unwanted effects [Citation24,Citation25]. In the future, advances in imaging, such as identification of high-risk regions with amino acid-PET, potentially help direct high doses to only precisely defined areas, thus exploiting the potential of dose escalation with particle therapy, but minimizing the risk for treatment-associated side effects [Citation26–29].
Studies implementing comparable doses of radiation for high-grade gliomas have yet not been reported, and data from low-grade gliomas have shown comparable outcome to photon treatment [Citation30]. However, in these patients the main benefit of protons may be in reduction of neurocognitive sequelae, and from most series no long-term follow-up has been reported, or may not include prospective assessment of neurocognitive scores. In the present series, we see promising local control rates, and no severe treatment-related side effects. Acute toxicity may be reduced compared to photons regarding fatigue, etc., however, hair loss is also present in the majority of the patients. In Japan, a smaller series of patients was treated with carbon ion radiotherapy for low-grade gliomas; progression free survival was at a median of 18 months in patients treated with lower doses (50.4 Gy E), and 91 months in the higher dose group (55.2 Gy E); it could be shown that dose had a significant impact on outcome local control. Toxicity was acceptable, no Grade III side effects were observed during the follow-up time [Citation31]. However, due to the biology of carbon ions and the natural behavior of low-grade gliomas, a clear rationale for carbon ions might be difficult, however, when available, potentially the superior dose distribution compared to protons and the RBE might convert to a clinical benefit, especially in patients with tumors close to OAR, or with adverse prognostic factors.
Meningiomas have been a target for proton centers in the past; again, for low-grade meningiomas the rationale is most likely reduction of long-term side effects, while high-risk meningiomas may benefit from dose escalation with particles. For this purpose, we treated 10 patients with high-grade meningiomas with photon radiotherapy up to 50 Gy E with a carbon ion boost of 18 Gy E to the macroscopic tumor [Citation32]. Two patients of 10 developed tumor recurrence after re-irradiation, six and 67 months after treatment. Local control rates after primary RT was 86% and 72% at five and seven years, respectively, which compared favorably to data in the literature. However, it is not clear whether this is due to the biology of carbon ions, or the dose escalation compared to conventional series treated commonly up to 60 Gy E; for high-grade meningiomas, again, a dose-response relationship is known, and doses exceeding 60 Gy E are anticipated to further increase local control [Citation33–36]. For low-grade meningiomas, again, studies to date have not shown superior results to photons, however, long-term assessment is still to be awaited. Currently, a prospective trial on high-risk atypical meningiomas is further assessing the role of a carbon ion boost [Citation37]. Target volume definition is based on molecular imaging using e.g. 68-Ga-DOTATOC-PET, which has been shown to have significant impact on identification of target lesions, as well as on target volume definition [Citation38,Citation39].
For recurrent tumors, the benefit or particle dose distributions underline the rationale for re-irradiations. Within our GSI experience, we could demonstrate safe and effective re-treatment using carbon ion radiotherapy in different anatomical regions [Citation40]. Currently, the value of carbon ion radiotherapy is compared to FSRT in a randomized controlled trial for recurrent glioblastomas [Citation23].
In conclusion, the data presented in the present manuscript add valuable information on patients treated with particle therapy within the brain and skull base region. Optimization in particle beam technology as well as advances in treatment planning can help improve outcome, especially in those patients with dismal prognosis such as glioblastomas or high-risk meningiomas. Currently, prospective trials are under way, thus the data on the value of particle therapy is enlarged continuously. Until these data are available, information on treatment concepts, toxicity and outcome as reported in the present analysis provide useful information for treating and referring physicians, and especially for those setting up a particle therapy service.
Acknowledgments
We thank Sabine Kuhn and her team of technicians for excellent patient care.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Part of this work was funded within Klinische Forschergruppe Schwerionentherapie KFO 214, as well as by the Dietmar Hopp Foundation. Clinical data were presented at the BIGART 2013 Meeting in Aarhus, Denmark, June 2013.
References
- Combs SE, Adeberg S, Dittmar JO, Welzel T, Rieken S, Habermehl D, et al. Skull base meningiomas: Long-term results and patient self-reported outcome in 507 patients treated with fractionated stereotactic radiotherapy (FSRT) or intensity modulated radiotherapy (IMRT). Radiother Oncol 2013;106:186–91.
- Combs SE, Bischof M, Welzel T, Hof H, Oertel S, Debus J, et al. Radiochemotherapy with temozolomide as re-irradiation using high precision fractionated stereotactic radiotherapy (FSRT) in patients with recurrent gliomas. J Neurooncol 2008;89:205–10.
- Combs SE, Behnisch W, Kulozik AE, Huber PE, Debus J, Schulz-Ertner D. Intensity modulated radiotherapy (IMRT) and fractionated stereotactic radiotherapy (FSRT) for children with head-and-neck-rhabdomyosarcoma. BMC Cancer 2007;7:177.
- Combs SE, Thilmann C, Debus J, Schulz-Ertner D. Local radiotherapeutic management of ependymomas with fractionated stereotactic radiotherapy (FSRT). BMC Cancer 2006;6:222.
- Combs SE, Gutwein S, Thilmann C, Debus J, Schulz-Ertner D. Reirradiation of recurrent WHO grade III astrocytomas using fractionated stereotactic radiotherapy (FSRT). Strahlenther Onkol 2005;181:768–73.
- Combs SE, Volk S, Schulz-Ertner D, Huber PE, Thilmann C, Debus J. Management of acoustic neuromas with fractionated stereotactic radiotherapy (FSRT): Long-term results in 106 patients treated in a single institution. Int J Radiat Oncol Biol Phys 2005;63:75–81.
- Bauman GS, Sneed PK, Wara WM, Stalpers LJ, Chang SM, McDermott MW, et al. Reirradiation of primary CNS tumors. Int J Radiat Oncol Biol Phys 1996;36:433–41.
- Combs SE, Edler L, Rausch R, Welzel T, Wick W, Debus J. Generation and validation of a prognostic score to predict outcome after re-irradiation of recurrent glioma. Acta Oncol 2013;52:147–52.
- Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: Long-term results in 172 patients treated in a single institution. J Clin Oncol 2005;23:8863–9.
- Athar BS, Paganetti H. Comparison of second cancer risk due to out-of-field doses from 6-MV IMRT and proton therapy based on 6 pediatric patient treatment plans. Radiother Oncol 2011;98:87–92.
- Paganetti H. Assessment of the risk for developing a second malignancy from scattered and secondary radiation in radiation therapy. Health Phys 2012;103:652–61.
- Paganetti H, Athar BS, Moteabbed M, Adams A, Schneider U, Yock TI. Assessment of radiation-induced second cancer risks in proton therapy and IMRT for organs inside the primary radiation field. Phys Med Biol 2012;57:6047–61.
- Yock TI, Caruso PA. Risk of second cancers after photon and proton radiotherapy: A review of the data. Health Phys 2012;103:577–85.
- Mizoe JE, Tsujii H, Hasegawa A, Yanagi T, Takagi R, Kamada T, et al. Phase I/II clinical trial of carbon ion radiotherapy for malignant gliomas: Combined x-ray radiotherapy, chemotherapy, and carbon ion radiotherapy. Int J Radiat Oncol Biol Phys 2007;69:390–6.
- Schulz-Ertner D, Nikoghosyan A, Hof H, Didinger B, Combs SE, Jakel O, et al. Carbon ion radiotherapy of skull base chondrosarcomas. Int J Radiat Oncol Biol Phys 2007; 67:171–7.
- Schulz-Ertner D, Karger CP, Feuerhake A, Nikoghosyan A, Combs SE, Jakel O, et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys 2007;68:449–57.
- Elsasser T, Kramer M, Scholz M. Accuracy of the local effect model for the prediction of biologic effects of carbon ion beams in vitro and in vivo. Int J Radiat Oncol Biol Phys 2008;71:866–72.
- Elsasser T, Scholz M. Improvement of the local effect model (LEM) – implications of clustered DNA damage. Radiat Prot Dosimetry 2006;122:475–7.
- Kramer M, Scholz M. Treatment planning for heavy-ion radiotherapy: Calculation and optimization of biologically effective dose. Phys Med Biol 2000;45:3319–30.
- Schlampp I, Karger CP, Jakel O, Scholz M, Didinger B, Nikoghosyan A, et al. Temporal lobe reactions after radiotherapy with carbon ions: Incidence and estimation of the relative biological effectiveness by the local effect model. Int J Radiat Oncol Biol Phys 2011;80:815–23.
- Fitzek MM, Thornton AF, Rabinov JD, Lev MH, Pardo FS, Munzenrider JE, et al. Accelerated fractionated proton/ photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: Results of a phase II prospective trial. J Neurosurg 1999;91:251–60.
- Combs SE, Kieser M, Rieken S, Habermehl D, Jakel O, Haberer T, et al. Randomized phase II study evaluating a carbon ion boost applied after combined radiochemotherapy with temozolomide versus a proton boost after radiochemotherapy with temozolomide in patients with primary glioblastoma: The CLEOPATRA trial. BMC Cancer 2010; 10:478.
- Combs SE, Burkholder I, Edler L, Rieken S, Habermehl D, Jakel O, et al. Randomised phase I/II study to evaluate carbon ion radiotherapy versus fractionated stereotactic radiotherapy in patients with recurrent or progressive gliomas: The CINDERELLA trial. BMC Cancer 2010;10:533.
- Rieken S, Habermehl D, Haberer T, Jaekel O, Debus J, Combs SE. Proton and carbon ion radiotherapy for primary brain tumors delivered with active raster scanning at the Heidelberg Ion Therapy Center (HIT): Early treatment results and study concepts. Radiat Oncol 2012;7:41.
- Rieken S, Habermehl D, Nikoghosyan A, Jensen A, Haberer T, Jakel O, et al. Assessment of early toxicity and response in patients treated with proton and carbon ion therapy at the Heidelberg ion therapy center using the raster scanning technique. Int J Radiat Oncol Biol Phys 2011;81: e793–801.
- Grosu AL, Astner ST, Riedel E, Nieder C, Wiedenmann N, Heinemann F, et al. An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys 2011;81:1049–58.
- Grosu AL, Weber WA, Franz M, Stark S, Piert M, Thamm R, et al. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys 2005;63:511–9.
- Grosu AL, Weber WA, Riedel E, Jeremic B, Nieder C, Franz M, et al. L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys 2005;63:64–74.
- Gumprecht H, Grosu AL, Souvatsoglou M, Dzewas B, Weber WA, Lumenta CB. 11C-Methionine positron emission tomography for preoperative evaluation of suggestive low-grade gliomas. Zentralbl Neurochir 2007;68:19–23.
- Hauswald H, Rieken S, Ecker S, Kessel KA, Herfarth K, Debus J, et al. First experiences in treatment of low-grade glioma grade I and II with proton therapy. Radiat Oncol 2012;7:189.
- Hasegawa A, Mizoe JE, Tsujii H, Kamada T, Jingu K, Iwadate Y, et al. Experience with carbon ion radiotherapy for WHO Grade 2 diffuse astrocytomas. Int J Radiat Oncol Biol Phys 2012;83:100–6.
- Combs SE, Hartmann C, Nikoghosyan A, Jakel O, Karger CP, Haberer T, et al. Carbon ion radiation therapy for high-risk meningiomas. Radiother Oncol 2010;95:54–9.
- Adeberg S, Hartmann C, Welzel T, Rieken S, Habermehl D, von Deimling A, et al. Long-term outcome after radiotherapy in patients with atypical and malignant meningiomas – clinical results in 85 patients treated in a single institution leading to optimized guidelines for early radiation therapy. Int J Radiat Oncol Biol Phys 2012;83:859–64.
- Engenhart-Cabillic R, Farhoud A, Sure U, Heinze S, Henzel M, Mennel HD, et al. Clinicopathologic features of aggressive meningioma emphasizing the role of radiotherapy in treatment. Strahlenther Onkol 2006;182:641–6.
- Katz TS, Amdur RJ, Yachnis AT, Mendenhall WM, Morris CG. Pushing the limits of radiotherapy for atypical and malignant meningioma. Am J Clin Oncol 2005; 28:70–4.
- Pasquier D, Bijmolt S, Veninga T, Rezvoy N, Villa S, Krengli M, et al. Atypical and malignant meningioma: Outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the rare cancer network. Int J Radiat Oncol Biol Phys 2008;71:1388–93.
- Combs SE, Edler L, Burkholder I, Rieken S, Habermehl D, Jakel O, et al. Treatment of patients with atypical meningiomas Simpson grade 4 and 5 with a carbon ion boost in combination with postoperative photon radiotherapy: The MARCIE trial. BMC Cancer 2010;10:615.
- Afshar-Oromieh A, Giesel FL, Linhart HG, Haberkorn U, Haufe S, Combs SE, et al. Detection of cranial meningiomas: Comparison of (6)(8)Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur J Nucl Med Mol Imaging 2012;39: 1409–15.
- Combs SE, Welzel T, Habermehl D, Rieken S, Dittmar JO, Kessel K, et al. Prospective evaluation of early treatment outcome in patients with meningiomas treated with particle therapy based on target volume definition with MRI and (68)Ga-DOTATOC-PET. Acta Oncol 2013;52:514–20.
- Combs SE, Kalbe A, Nikoghosyan A, Ackermann B, Jakel O, Haberer T, et al. Carbon ion radiotherapy performed as re-irradiation using active beam delivery in patients with tumors of the brain, skull base and sacral region. Radiother Oncol 2011;98:63–7.