Abstract
Background. On the basis of our own experience and literature search, we hypothesised that a canine olfactory test may be useful for detecting lung cancer in an unselected population of patients suspected to have lung cancer. Material and methods. We conducted a prospective study of 93 patients consecutively admitted to hospital with suspected lung cancer. Exhaled breath and urine were sampled before the patients underwent bronchoscopy. The canine olfactory test was performed in a double-blinded manner. Sensitivity and specificity were outcome measures. Results. With 99% sensitivity, the olfactory test demonstrated that dogs have the ability to distinguish cancer patients from healthy individuals. With an intensified training procedure, the exhaled breath and urine tests showed sensitivity rates of 56–76% and specificity rates of 8.3–33.3%, respectively, in our heterogeneous study population. Conclusion. Although the olfactory test appears to be a promising tool for the detection of cancer, the main challenge is to determine whether the test can sufficiently discriminate between patients at risk, patients with benign disease, and patients with malignant disease. We need to gain a deeper understanding of this test and further refine it before applying it as a screening tool for lung cancer in clinical settings.
Lung cancer is the number one cause of cancer death in industrialised countries [Citation1], and is an emerging global challenge, given the increase in smoking behaviour worldwide. Early detection followed by surgical resection of lung tumour produces the best long-term survival or cure. However, as this is applicable only in 15–20% of the lung cancer population, early screening detection programmes that enhance expensive modern imaging modalities are strongly warranted [Citation2]. Computed tomography (CT) screening have recently shown a long desired reduction in mortality, but no convincing evidence on the cost-effectiveness of radiological screening has been produced, and so far it is unknown [Citation3]. Behavioural risk factor interventions like smoking cessation have, so far, been the most effective methods in fighting lung cancer.
Much effort has been exerted worldwide in the development of simple, inexpensive screening tools for detecting lung cancer at an early, curable stage. During the last three decades, the utility of chromatography methods or so-called “electronic nose” methods for detecting cancer have been studied intensively; however, these methods have yet to meet clinical expectations [Citation4–6]. Novel approaches for detection have begun to be explored.
Dogs have an extremely acute sense of smell, enabling them to discriminate between virtually innumerable scent qualities. This “PET scan” has been used widely in civilian rescue services and at crime scenes to enhance detection of humans and contraband [Citation7,Citation8]. The olfactory test (i.e. dogs’ ability to discriminate scents) has emerged as a promising tool for the screening and early detection of different kinds of cancer in humans [Citation9–13]. In 2006 McCulloch et al. evaluated an olfactory test for detecting lung cancer in which exhaled breath was “analysed” by ordinary household dogs. The test had sensitivity and specificity rates of 99%, and the results were independent of the clinical stage of disease [Citation10]. Horvath et al. showed that trained dogs could detect ovarian carcinoma by sniffing tumour tissue and blood samples. The sensitivity rate was 100% for both sample types, and the specificity rate was 95% and 98%, respectively [Citation11]. Cornu et al. reported that trained dogs could detect prostate cancer by sniffing urine, at sensitivity and specificity rates of 91% [Citation12]. These studies demonstrate that dogs are capable of discriminating patients with cancer from presumptive healthy people.
In 2012 Ehmann and colleagues used dogs to assess the exhaled breath of healthy people, COPD patients, and lung cancer patients [Citation13], making the test more challenging. Although the test's sensitivity rate decreased to 70%, it still had a high specificity rate of 93%. The authors concluded that a stable marker or scent pattern associated with lung cancer probably exists and that this scent signature is likely not present in COPD. In addition, they observed that the dogs were able to discriminate tobacco smoke, food odours, and potential drug metabolites [Citation13]. As with McCulloch et al., Ehmann et al. proposed further studies to address the following question: Can sniffer dogs discriminate benign lung lesions from lung cancer?
With the aim of using scent dogs as a screening tool in the clinic, we hypothesised that dogs can discriminate patients with malignant disease from those with different benign conditions. To test this hypothesis, we conducted a clinically relevant study of unselected patients admitted to hospital with suspected lung cancer. At the time of inclusion in our study, none of the patients were healthy.
At the Trondheim School of Dog Behaviour, teachers and handlers have a special interest in training and using behavioural modification techniques for dogs. Besides having olfactory test programmes for different purposes, this group is particularly interested in the detection of cancer, especially lung cancer. Thus, an olfactory test was developed. On the basis of our own pilot experience with the test method and knowledge from the literature, we conducted the present study.
Material and methods
Subjects
The study population included 93 consecutive patients with suspected lung cancer, all benign or malignant lung disease; none were healthy at the time of inclusion. Patients that were unable to perform the exhaled breath test were not included. Patients testing negative for malignant lung disease were followed up for three years in order to detect any future occurrence of cancer. The study was performed between June 2006 and January 2009.
Diagnosis of lung cancer
The diagnosis of lung cancer was based on the classification system established by the World Health Organization. Diagnosis was histologically verified from biopsy or cytology specimens [Citation14]. Confirmed malignant diagnoses were categorised into four groups: 1) non-cancer; 2) small-cell lung cancer (SCLC); 3) non-small cell lung cancer (NSCLC); and 4) non-lung cancer (pulmonary carcinoid, mesothelioma or lung metastasis from other primary neoplasms). We used the term “not confirmed” for tumours that clinically and radiologically determined to be lung cancer and treated as such, but were not histologically verified for various reasons.
Sampling of exhaled breath and urine
Both exhaled breath and urine used for the olfactory test were collected from the subjects before performing bronchoscopy and at least four hours after oral intake or tobacco smoking (range 4–12 hours). The patients exhaled air (three forced expirations) through sterile exhalation filters (Breating Filter Allegro ID 547468, Allegro Medical Inc, US), and the filters were transferred to cool storage in specially cleaned and sealed containers. The filters were placed into these containers at room temperature one hour before the olfactory test.
Urine (10 ml) was collected, stored in cryotubes, and stored at –20oC. Urine samples were thawed to room temperature one hour before the olfactory test. Both the exhaled air containers and thawed urine (1–2 droplets in sterile a cryotube) were immediately put into separate specially sealed glass containers to eliminate possible mixing of different odour compounds. The containers were labelled with a code number with reference to the patient, date of test, and type of test material.
Finally, the exhaled breath and urine samples were transported to the laboratory at Trondheim School of Dog Behaviour for scent dog testing. Exhaled breath samples were stored at room temperature in the specially sealed containers, and the urine was frozen at –20oC. Trained laboratory staff let the dogs perform the olfactory test as soon as the exhaled breath samples arrived. The olfactory test took place no later than 2–4 weeks after sampling; for urine samples, the test took place within hours after thawing the frozen samples. Urine samples were thawed up and the olfactory test was performed (first urine test, after basic training), and re-frozen before it was thawed up again for the second urine test (after intensified training) 6–30 months after the time for collection (June–November 2009), dependent on the time of inclusion and the time interval to test performance. Even though it was not the scope of this study, the test quality in terms of durability was tested both for exhaled breath (contaminated filters) and urine. The sensitivity was kept at 99% at intervals of 6, 12, 18, and 24 months, using the same positive control and study material. There was complete agreement among the repeated test results for all available dogs (data not shown).
Positive control (lung cancer tumours)
Lung cancer tissue from preoperative tumour biopsies or resected lung tumours was used as positive control samples for training the dogs. Dogs were exposed to particles of the actual tumour or to a scent imprint (contamination) on sterilised cotton wool pads. The positive control was either smaller cutting particles of few millimetres (fresh parts of the tumour) which were put into a metal net for 15 minutes in a sealed container with cotton wool pads, or succulent imprints from sections of the same lung tumour adapted on a sterile cotton wool pad. Both types of tumour materials were found to be equally effective when pilot tested in our laboratory (sensitivity 99%). The test material was the same for all dogs participating in a test period for a defined patient group and period. We had access to two lung tumours, a small cell lung cancer and a non-small cell lung cancer. Only one of them was used for training and testing, including all the dogs, for a specific group of patients and period. Tumour tissue was frozen at –28oC. Whenever the tumour tissue was needed, it was brought to room temperature within one hour in order to obtain “fresh” material for positive test training. The positive control material was used only once.
Urine from patients with known lung cancer was also collected and tested as a positive control. Urine had the same 99% sensitivity as exhaled breath and was used in the training period before the first and second olfactory urine test of the subject population.
Negative control
Exhaled breath and urine samples from 20 presumably healthy young persons were used as control test material for training the dogs. The samples were prepared using the same equipment and procedure as positive test samples taken from lung cancer patients.
Training of dogs
Training was conducted at the Trondheim School of Dog Behaviour using an established procedure for scent training of dogs, and the training did not vary among the dogs. Firstly, the dogs learned the odour signature (identified tumour odour at low concentrations). Secondly, the dogs learned to discriminate odour qualities (i.e. between tumour odour and healthy odour). The dogs, however, were not trained to distinguish extraneous odours. In order to maintain olfactory test sensitivity at 99% for both exhaled breath and urine samples, dogs underwent at least two training sessions per week for the duration of the study.
Positive (lung tumour tissue) and negative control samples were placed randomly in roundels containing six holders. The number of control samples in the roundels varied from 0–6 samples. If the dogs were used in between the two training sessions for tasks other than those involving our study, they were trained for at least four weeks before the next test session. This was necessary in order to maintain olfactory test sensitivity at 99%.
Interim analysis of the first 46 patients indicated that accuracy rates were low. Consequently, we decided to intensify training by increasing the number of weekly training sessions to four per week for each dog.
The olfactory test
Exhaled breath or urine samples from the subjects were randomly placed in a roundel. The exhaled breath and urine samples were tested separately. Thus, the roundel consisted of samples from patients with either cancer or other lung diseases. The number of cancer samples in the rondels varied from 0–6 samples. The dogs were kept in separate rooms until the test was performed. For the test, each dog was allowed to pass by the test containers. The dog's behaviour was observed by an experienced dog trainer, who was positioned 3–4 m away or behind a screen. Both the trainer and dogs did not know what was in the containers. Hence, the test was double blind. Further, the study was randomised, as prospective inclusion of patients with lung cancer or benign condition led to a random number and placement of cancer and benign samples in the roundel.
A positive test was manifested in a variety of ways, some dogs stopped and remained standing over the sample (eyeing it, scratching it with one paw, or freezing), while others either sat or lay down in front of it (). A negative test response was manifested differently: The dogs just passed by the samples without making any particular sign. Each dog had a unique marking behaviour. The unique behaviour pattern or response, either it was on the positive or the negative test, was more the result of a natural response pattern expressed by each of the dogs than a consequence of a specific learning procedure for each dog. The response pattern was well-known by the experienced dog trainer. Search mode: the dog moved in steady pace forward with fixed sniffing pattern. Indications (either negative or positive): negative, the dog passed the test sample without change of pace, and positive, the dog stopped forward movement and displaying specific fixed indication behaviour.
Figure 1. Olfactory test showing the different dogs’ marking behaviour. Shown is a plank or roundel containing a random order of positive (tumour) and negative samples (no tumour). A, Jippi; B, Fröya; C, Kaos; and D, Tassen.
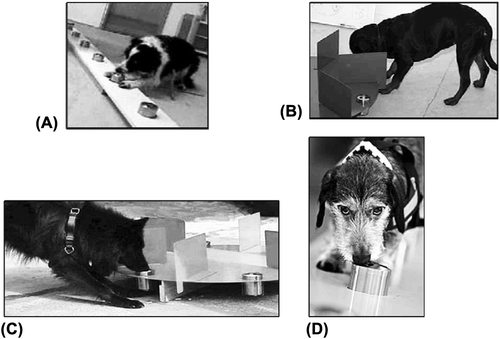
The test was video-taped and controlled to assure that the correct reaction was registered. The handlers were convinced that the interpretations on-site during test conditions and the interpretations of the videos were correct, and both were unanimous. If two or more dogs displayed a positive test response, the sample was regarded positive for lung cancer, and if two or more dogs displayed a negative test response, the sample was regarded negative for lung cancer.
Dog characteristics
The dogs were named Kaos, Jippi, Tassen, and Fröya (), and their characteristics are listed in . Initially, the study used three dogs. However, one of the dogs, Tassen, became sick 2.5 years into the study. Thus, a new dog, Fröya, was trained to replace the sick dog. This delayed the study for approximately six months.
Table I. Characteristics of the four dogs.
Endpoints
The primary outcome measures were the sensitivity and specificity of a dog's ability to detect lung cancer from exhaled breath or urine samples taken from patients with suspected lung cancer compared to the confirmed histopathological diagnosis. We also compared the results of the olfactory breath test and the olfactory urine test.
The present study aimed to evaluate the olfactory test as a proof-of-principle study in a clinically meaningful setting and to compare it to standard diagnostic methods.
Statistics
The statistical software SPSS WP 17 (SPSS Inc., 2008) [Citation15] was used for descriptive statistics and for calculating frequencies, and sensitivity and specificity rates. Sensitivity (true-positive rate) was calculated as the proportion of cancer samples correctly identified by the dog, and specificity (true-negative rate) was calculated as the proportion of control samples found by the dog to be negative for cancer. Sensitivity and specificity were used to describe the accuracy of the tests. The use of binominal probability distribution led to the ordinary definition of sensitivity as a/(a + c) and specificity as d/(b + d).
Ethical considerations
The Regional Ethics Committee for Research in Medicine approved the study, which included written and spoken informed consent from the subjects. The dogs were handled with good care in agreement with the Helsinki convention for the use and care of research animals, in a certified training centre with well-trained personnel.
Results
Demographic data of the study population
Characteristics of the malignancy, smoking habits, concomitant disease, and lung function ( and ) confirm that the study population was representative for our population. During the study 93 unselected patients with pulmonary infiltrates and suspected lung cancer were enrolled. The study population was a heterogeneous population in terms of lung disorders.
Table II. Characteristics of the malignancy in the study population.
Table III. Smoking history and concomitant disease in the study population.
The median subject age was 70 years (range 44–89 years) for the cancer group and 67 years (range 44–84 years) for the non-cancer group. The overall prevalence of cancer diagnosis (lung cancer and lung metastasis from other solid tumours) was 67.7% (63 of 93 patients). Of these, 59 (63.4%) had lung cancer and four (4.3%) experienced lung metastasis from other solid tumours (glioblastoma, n = 1; uterine cancer, n = 1; and urinary bladder cancer, n = 2). The spirometry results showed FVC 3.5 (± 1.1) L (SD)/87.8 (17.9) % predicted and FEV1 2.2 (± 0.7) L (SD)/72.7 (17.5) % predicted (SD) for the patients with malignancy in lung, and FVC 3.7 (± 1.2) L (SD)/83.1 (14.3) % predicted (SD) and FEV1 2.3 (± 1.1) L (SD)/67.7 (23.1) % predicted (SD) in the group with no malignancy in lung.
Other extrapulmonary solid cancers were found in eight subjects (13.8%) in the lung cancer group and in one subject (3.3%) in the non-cancer group (). Other concomitant diseases were almost equally distributed in both groups. All included subjects were able to sufficiently perform the exhaled breath test. In order to follow the test procedure for different groups of patients during the study period, the shows a structured presentation of the specific patient population that was tested with the specific test material along the time course of the study.
Table IV. Table showing structured presentation of the specific patient population that was tested with the specific test material along the time course of the study.
Exhaled breath test
Interim analysis of the first 46 patients was performed in order to evaluate the olfactory breath test and in order to refine the study protocol if necessary. The prevalence of lung cancer in this group was 71%. Prior to testing, the dogs had undergone an ordinary (maintenance) training session. The results of the exhaled breath test for each dog (Kaos, Jippi, Tassen), as well as the overall results, is presented in . Sensitivity and specificity for NSCLC were 70% and 8.3%, respectively; and sensitivity and specificity for SCLC were 55.6% and 8.3%, respectively.
Table V. Table summarizing all olfactory test results: First exhaled breath test (First EBT) and detection of lung cancer after basic training: results for each dog and overall results for the first 46 patients. Second exhaled breath test (Second EBT) and detection of lung cancer after intensified training: the last 40 patients. First and second urine test (First UT and Second UT) and detection of lung cancer after basic training and intensified training, respectively: results of samples for 77 patients.
After the interim analysis, we decided to continue the study with another 29 patients, which resulted in nearly the same results as with the first 46 patients (data not shown). As these results were below our expectations, we decided to train the dogs more intensively and to aim for an “intensive training” effect in the next round of analyses. At this point, Tassen became ill and had to be replaced with Fröya. After the intensive training, the last 40 patients were tested (). Lung cancer prevalence in this group was 73%. Sensitivity and specificity for NSCLC were 60% and 33.3%, respectively; and sensitivity and specificity for SCLC were 100% and 33.3%, respectively. After intensive training, improvement was observed only for the SCLC group.
Urine test
Urine testing () was performed after all the exhaled breath samples were tested and in the same manner as with the exhaled breath test. The first urine test was done after ordinary training. The urine of only 77 of the 93 patients was tested, as 16 patients were unable to deliver the required sample volume of urine for two consecutive tests. The lung cancer prevalence in this group was 71%. The test panel consisted of three dogs, Kaos, Fröya, and Jippi. Analysing the olfactory test results for NSCLC specifically overall sensitivity and specificity were 65.7% and 25%, respectively; and the overall sensitivity and specificity for SCLC were 90% and 25%, respectively.
After the dogs underwent intensive training, a second urine test was performed using samples from the same 77 patients (). Sensitivity and specificity for NSCLC were 60% and 29.2%, respectively; and sensitivity and specificity for SCLC were 80% and 29.2%, respectively.
Discussion
To the best of our knowledge, the present study is the first to use the olfactory test to detect lung cancer in unselected patients suspected to have lung cancer. Previous reports have shown that the olfactory test does well in discriminating cancer patients from presumptive healthy people. However, recent studies have shown that it is less valuable in patients with comorbidities like COPD [Citation13].
In our laboratory, olfactory pilot tests had an accuracy level of 99% for both exhaled breath and urine when used to discriminate cancer patients from presumptive healthy individuals, hence confirming that our test worked. The olfactory test demonstrated that the dogs were able to determine whether a person was sick or healthy (training conditions). The dogs, however, could not sufficiently discriminate between malignant and benign conditions with the current training protocol. The exhaled breath test had a sensitivity rate that varied from 56% to 76% across the different dogs and a specificity rate that varied from 8.3% to 33.3%. These results indicate that this test, as currently developed, is inappropriate for in-clinic use, especially because of its inability to rule out lung cancer (i.e. false negatives). This rather low sensitivity is consistent with the results of the Ehmann study (which reported a sensitivity of 70%) that included cancer patients, COPD patients, and healthy individuals. Taken together, these comparable sensitivity results may indicate that the olfactory test is more likely to discriminate sick patients from presumptive healthy people, but not patients with benign disease from patients with malignant disease.
The performance gap in the olfactory test's ability to distinguish between cancer and healthy states and between malignant and benign conditions presents challenges for using this test in clinical contexts. There are several possible explanations for this performance difference. The specificity of the “odour signature” associated with different conditions, other than those associated with healthy people, may be low. Also, samples may contain innumerable “specific scent qualities” that are associated with the tumour and the host (e.g. scents associated with food, medication and tobacco smoking). Thus, numerous unknown variables could differentially contribute to performance in the two situations. The odour signature from different cancers may most probably appear differently, but one could also think of the possibility of different scent qualities based on the different histological subgroups in lung cancer or within the group of NSCLC. We may be sceptic to what would happen if the dogs were trained with SCLC (positive control) and the actual patient had NSCLC, or similarly what if an adenocarcinoma positive test was used in a person with squamous cell carcinoma. Still we do not know whether histo-pathological subgroups have a selection of common scent qualities or whether there are specific scent markers for lung cancer in general. There may also be strong and disturbing “non-malignant odours” that may suppress the sense of “malignant odours”. At the present time there are sparse knowledge and experience to provide qualified answers these questions. The dogs’ ability to scent cancer may even be too good, so that the dogs may register too many odours or qualities of cancer, hence they may be difficult to be distinguished. This will be further complicated when best reproducibility is required and warrants highly qualified maintenance training. Another challenge for the olfactory test is how to maintain the “in-house” level of accuracy. The dog is a living creature that gets hurt and may be influenced by intercurrent infections or allergies, which may reduce the quality of the olfactorial test conditions.
Although the exhaled breath test conducted after intensified dog training and the first urine test showed slightly higher specificities, it was still not clinically useful. A training effect must be carried out under comparable experimental conditions before and after training, which may not have been the case in this study. We had access to both NSCLC and SCLC, which we used as positive control samples during the dogs’ training. Either NSCLC or SCLC samples were presented to the dogs randomly (either one or the other) as “lung cancer positive control”. That may explain the small differences in test results before and after training when comparing NSCLC and SCLC.
The more specific the positive test is for lung cancer, the better the test will work. Ideally, the only difference should be the only single molecule that alone may characterise the specific condition and be detected by the dog. At the moment, a selective positive test for lung cancer does not exist. As more refined technical instruments continue to be developed for laboratory use, these advanced instruments may soon be able to detect and sort out volatile organic compounds (VOCs) unique to specific cancers. But the issue of economic feasibility remains with future diagnostics.
If certain VOCs specific for a condition of interest (chemical signatures) are also water soluble, theoretically, they could be detected and identified in exhaled breath condensate (EBC) or urine by using mass spectrometry and colorimetric methods [Citation16,Citation17]. A variety of techniques have been used to analyse different compounds in EBC. For instance, chemiluminiscence has been used to detect NO, cytokines, DNA, and oxidative stress markers, and gas chromatography or mass spectrometry has been used to analyse VOC. The latter, which has been referred to “an electronic nose for molecule detection” is at present a hot topic. Regardless, it is still difficult to find the needle in the haystack when dealing with lung cancer diagnosis [Citation18,Citation19].
Other approaches are now being explored. Nanotechnology methods employing arrays of sensors based on gold nanoparticles have the capacity to rapidly differentiate the exhaled breath of lung cancer patients from that of healthy individuals with 86% accuracy [Citation20,Citation21]. Mice have recently been shown to have the ability to detect minute differences in the urinary odour of mice with cancer and healthy mice at accuracy values ranging from 94% to 100% [Citation22].
The recent mini-review by Lippi and Cervellin discusses theoretical and practical difficulties related to the olfactory test [Citation23]. It is difficult to compare different olfactory test studies because performance varies across different test dogs within the same study and between different studies; because different modalities are used to train the dogs; and because different types of cancer are investigated. Another problem associated with olfactory test research is that organ- and/or cancer-specific signatures may exist but may be masked by confounding and overlapping biochemical signals from inflammatory diseases, diet, and pharmaceuticals. Furthermore, they emphasised that the promising historical results were based on the discrimination of presumptive healthy people from cancer patients.
Lippi and Cervellin argue for more studies comparing patients with suspected lung cancer. The present study responds to this call, producing initial results to evaluate. The olfactory test method has shown to be promising in a large number of studies over a long period of time. It is our opinion that this method should be a topic for thorough research, as a deeper understanding at the molecular level and at the practical test level is necessary if the test is to be used successfully for clinical applications. The first step may be a more specific characterisation of tumours in terms of their specific scent qualities, specific molecules, or scent signatures, which can be further studied with the olfactory test or the electronic nose, or both [Citation24].
In conclusion, the olfactory test concept holds promise for use in the detection of cancer at an early stage. However, in the present study it failed to meet the level of specificity needed for in-clinic applications. Thus, we need to gain a deeper understanding of the olfactory test and to make specific refinements before it can be used as a clinical screening tool for lung cancer. Future research should focus on identifying the specific scent signatures of tumours and hosts, a necessary step for both the “electronic nose approach” and the olfactory test using hypersensitive domestic animals.
Acknowledgements
We want to thank Anne Stine Fossum, Inger Lise Bjerkan, Birgit Pedersen, and Randi Sailer, the nurses at the Department of Thoracic Medicine, St. Olavs Hospital, who contributed in the sampling, collection, and storing of bronchoscopy, blood, and urine samples. We also want to thank Siren Thorsvik (mathematician and statistician) for her contribution to the statistical analysis, and the dog handlers and Turid Buvik, Anne Marit Hagen and Mette Olsen at Trondheim School of Dog Behaviour.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
We thank the local cancer foundation, Kreftfondet St. Olavs Hospital, for providing support for the first year of this project.
References
- Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines, 2nd ed. Chest 2007;132(3 Suppl):29S–55S.
- Spiro SG, Navani N. Screening for lung cancer: Is this the way forward?. Respirology 2012;17:237–46.
- Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. New Engl J Med 2011;365:395–409.
- Peng G, Hakim M, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, et al. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br J Cancer 2010;103:542–51.
- Horvath I, Lazar Z, Gyulai N, Kollai M, Losonczy G. Exhaled biomarkers in lung cancer. Eur Respir J 2009;34: 261–75.
- D’Amico A, Pennazza G, Santonico M, Martinelli E, Roscioni C, Galluccio G, et al. An investigation on electronic nose diagnosis of lung cancer. Lung Cancer 2010;68: 170–6.
- Lorenzo N, Wan T, Harper RJ, Hsu YL, Chow M, Rose S, et al. Laboratory and field experiments used to identify Canis lupus var. familiaris active odor signature chemicals from drugs, explosives, and humans. Anal Bioanal Chem 2003; 376:1212–24.
- Gazit I, Lavner Y, Bloch G, Azulai O, Goldblatt A, Terkel J. A simple system for the remote detection and analysis of sniffing in explosives detection dogs. Behav Res Method Instrum Comp 2003;35:82–9.
- Balseiro SC, Correia HR. Is olfactory detection of human cancer by dogs based on major histocompatibility complex-dependent odour components? – A possible cure and a precocious diagnosis of cancer. Med Hypotheses 2006;66:270–2.
- McCulloch M, Jezierski T, Broffman M, Hubbard A, Turner K, Janecki T. Diagnostic accuracy of canine scent detection in early- and late-stage lung and breast cancers. Integr Cancer Ther 2006;5:30–9.
- Horvath G, Andersson H, Paulsson G. Characteristic odour in the blood reveals ovarian carcinoma. BMC Cancer 2010;10:643.
- Cornu JN, Cancel-Tassin G, Ondet V, Girardet C, Cussenot O. Olfactory detection of prostate cancer by dogs sniffing urine: A step forward in early diagnosis. Eur Urol 2011;59:197–201.
- Ehmann R, Boedeker E, Friedrich U, Sagert J, Dippon J, Friedel G, et al. Canine scent detection in the diagnosis of lung cancer: revisiting a puzzling phenomenon. Eur Respir J 2012;39:669–76.
- Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. WHO-classification of malignant tumours; tumors of the lung, pleura, thymus and heart. IARC Press: Lyon, France; 2004.
- SPSS-Inc. SPSS Statistics for Windows, Version 17.0, Chicago, US; SPSS Inc. 2008.
- Chan HP, Lewis C, Thomas PS. Exhaled breath analysis: Novel approach for early detection of lung cancer. Lung Cancer 2009;63:164–8.
- Mazzone PJ. Analysis of volatile organic compounds in the exhaled breath for the diagnosis of lung cancer. J Thorac Oncol 2008;3:774–80.
- Barta I, Kullmann T, Csiszer E, Antus B. Analysis of cytokine pattern in exhaled breath condensate of patients with squamous cell lung carcinoma. Int J Biol Marker 2010;25:52–6.
- Dragonieri S, van der Schee MP, Massaro T, Schiavulli N, Brinkman P, Pinca A, et al. An electronic nose distinguishes exhaled breath of patients with malignant pleural mesothelioma from controls. Lung Cancer 2012;75:326–31.
- Mazzone P. Nanomedicine: Sniffing out lung cancer. Nature Nanotechnol 2009;4:621–2.
- Peng G, Tisch U, Adams O, Hakim M, Shehada N, Broza YY, et al. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nature Nanotechnol 2009;4: 669–73.
- Matsumura K, Opiekun M, Oka H, Vachani A, Albelda SM, Yamazaki K, et al. Urinary volatile compounds as biomarkers for lung cancer: A proof of principle study using odor signatures in mouse models of lung cancer. PloS One 2010;5:e8819.
- Lippi G, Cervellin G. Canine olfactory detection of cancer versus laboratory testing: Myth or opportunity?Clin Chem Lab Med 2012;50:435–9.
- Boedeker E, Friedel G, Walles T. Sniffer dogs as part of a bimodal bionic research approach to develop a lung cancer screening. Interact Cardiovasc Thorac Surg 2012;14:511–5.

