Abstract
Background. Little information is available about survival outcomes of patients with HER2-positive early breast cancer treated with adjuvant capecitabine-containing chemotherapy with or without trastuzumab. Patients and methods. One thousand and five hundred patients with early breast cancer were entered to the Finland Capecitabine trial (FinXX) between January 2004 and May 2007, and were randomly assigned to receive either three cycles of adjuvant TX (docetaxel, capecitabine) followed by three cycles of CEX (cyclophosphamide, epirubicin, capecitabine; TX-CEX) or three cycles of docetaxel followed by three cycles of CEF (cyclophosphamide, epirubicin, fluorouracil; T-CEF). The primary endpoint was recurrence-free survival (RFS). The study protocol was amended in May 2005 while study accrual was ongoing to allow adjuvant trastuzumab for patients with HER2-positive cancer. Of the 284 patients with HER2-positive cancer accrued to FinXX, 176 (62.0%) received trastuzumab after amending the study protocol, 131 for 12 months and 45 for nine weeks. The median follow-up time was 6.7 years. Results. Patients with HER2-positive cancer who received trastuzumab had better RFS than those who did not (five-year RFS 89.2% vs. 75.9%; HR 0.41, 95% CI 0.23–0.72; p = 0.001). Patients treated with trastuzumab for 12 months or nine weeks had similar RFS. There was no significant interaction between trastuzumab administration and the type of chemotherapy. Four (2.3%) patients treated with trastuzumab had heart failure or left ventricular dysfunction, three of these received capecitabine. Conclusion. Adjuvant trastuzumab improves RFS of patients treated with TX-CEX or T-CEF. Few patients had cardiac failure.
Adjuvant trastuzumab improves disease-free survival [Citation1–5] and overall survival [Citation1,Citation5] of patients with HER2-positive breast cancer based on randomized clinical trials. The most important adverse effect of adjuvant trastuzumab is congestive heart failure [Citation6]. Heart failure was detected in 0.4–3.5% of patients in the major adjuvant trastuzumab trials [Citation7], but it may be more common than this in elderly populations [Citation8].
Little is known about the efficacy and safety of adjuvant trastuzumab when it is administered in combination with a capecitabine-containing chemotherapy regimen as compared with a regimen that does not contain capecitabine. Here we present the outcome data of patients with HER2-positive breast cancer treated with or without adjuvant trastuzumab within the context of the Finland Capecitabine Trial (FinXX). FinXX compared safety and efficacy of an adjuvant chemotherapy regimen that contained capecitabine (X), docetaxel (T), cyclophosphamide (C) and epirubicin (E, TX-CEX) to a regimen that did not contain capecitabine (T-CEF) [Citation9].
Patients and methods
Study design
FinXX is a randomized, prospective, phase III, open-label, multicenter trial. The results of the comparison between the chemotherapy regimens have been published after a median follow-up time of 4.9 years, and they tended to favor TX-CEX over T-CEF with five-year recurrence-free survival (RFS) of 86.6% and 84.1%, respectively, but this difference was not statistically significant (p = 0.087) [Citation9].
Patients
Women who had histologically confirmed invasive breast cancer with regional lymph nodes containing cancer, or node-negative cancer with primary tumor diameter > 20 mm and negative progesterone receptor (PR) expression in immunohistochemistry (usually defined as staining of < 10% of cancer cells) were eligible [Citation10]. Other key inclusion criteria were age 18 to 65 years; the World Health Organization (WHO) performance status < 2; the time interval between surgery and randomization ≤ 12 weeks; and normal hepatic, renal and cardiac function. Patients who had distant metastases at the time of study entry were excluded, as were patients who had node-negative mucinous, papillary, medullary or tubular cancer, and those who had clinically significant cardiac disease or who had received neoadjuvant chemotherapy. The study was conducted in accordance with the Helsinki Declaration, registered (www.ClinicalTrials.gov identifier NCT00114816), and the institutional review boards approved the study protocol. The patients provided written informed consent prior to study entry.
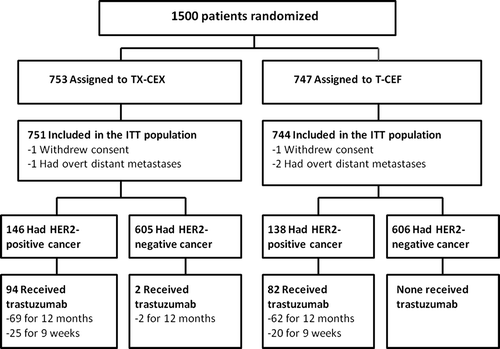
A total of 1500 patients entered the study between 27 January 2004 and 29 May 2007 [Citation9]. Two patients withdrew consent and three had overt distant metastases at staging, and were excluded from further analyses (). Of the 1495 remaining patients 284 (19.0%) had HER2-positive disease based on immunohistochemistry (HER2 expression classified as +++) or a positive in situ hybridization test at local assessment, and form the basis of the present analysis. The reproducibility of HER2 testing is generally good in the study regions [Citation11].
Following the release of the results of three randomized trials (HERA, the National Surgical Adjuvant Breast and Bowel Project trial B-31 and the North Central Cancer Treatment Group trial N9831) [Citation12,Citation13], the FinXX study protocol was amended in May 2005 while patient accrual was ongoing to allow treatment of patients with HER2-positive disease with adjuvant trastuzumab. At the time of the protocol amendment 619 (41.3%) of the planned 1500 patients had been entered the FinXX study. The study protocol was amended also in 27 May 2012 after publication of the study results based on a median follow-up time of 59 months in January 2012 [Citation9] to allow collection of further survival and safety data from the subgroup with HER2-positive disease and to achieve a longer follow-up time.
Study objectives
RFS (the primary endpoint) was defined as the time interval between the date of randomization and the date of detection of invasive breast cancer recurrence (local or distant), or death if the patient died prior to cancer recurrence. Second cancer or contralateral breast cancer were not counted as events. The secondary endpoints were cardiac adverse events and overall survival, defined as the time from the date of randomization to the date of death.
Adjuvant treatments
Patients were randomly assigned centrally in a 1:1 ratio to chemotherapy containing capecitabine and standard agents or to chemotherapy containing standard agents only [Citation9]. The patients assigned to capecitabine-containing chemotherapy received three cycles of TX (T, docetaxel; X, capecitabine) followed by three cycles of CEX (C, cyclophosphamide; E, epirubicin; X, capecitabine; TX × 3 → CEX × 3). TX consisted of docetaxel 60 mg/m2 given as a one-hour i.v. infusion on day 1 of every three-week cycle and capecitabine 900 mg/m2 given orally b.i.d. on days 1 to 15. CEX consisted of intravenous cyclophosphamide 600 mg/m2 and epirubicin 75 mg/m2 administered on day 1, and oral capecitabine 900 mg/m2 given b.i.d. days 1 to 15 every three weeks [Citation9]. Patients assigned to the control group received three cycles of docetaxel (80 mg/m2 administered as a one-hour i.v. infusion on day 1 of every three-week cycle) followed by three cycles of CEF (cyclophosphamide 600 mg/m2, epirubicin 75 mg/m2 and fluorouracil 600 mg/m2 administered on day 1 of each week-week cycle; T × 3 → CEF × 3). Chemotherapy was given for six cycles unless intolerable toxicity occurred or disease recurrence was detected. Chemotherapy doses were modified based on observed toxicity [Citation9].
Since the optimal method to administer trastuzumab was unknown, the method was not specified when the protocol was amended in 2005. Of the 284 patients with HER2-positive disease entered, 108 (38.0%) did not receive adjuvant trastuzumab [99 (91.7%) of these 108 patients initiated adjuvant treatment before 27 May 2005] and 176 (62.0%) received it.
Most patients with HER2-positive disease who were treated with adjuvant trastuzumab received it for 12 months [131(74.4%) of 176]. After the release of the FinHer trial results in December 2005 [Citation3], 45 (25.6%) patients received trastuzumab for nine weeks, administered as in the FinHer trial weekly and concomitantly with docetaxel [Citation3]. Most [n = 104 (79.4%)] patients who received trastuzumab for 12 months had all trastuzumab given after completion of chemotherapy administered as in the HERA trial [Citation12], whereas 27 (20.6%) patients received trastuzumab first for nine weeks concomitantly with docetaxel (administered as in the FinHer trial) and subsequently three-weekly for 42 weeks after chemotherapy to complete one year of administration (a loading dose of 8 mg/kg, then a dose of 6 mg/kg three-weekly). Two patients with HER2-negative cancer received adjuvant trastuzumab, since their cancer was first misdiagnosed as HER2-positive ().
Radiation therapy was given according to the institution's practice after completion of chemotherapy. Patients with steroid hormone receptor-positive disease [estrogen receptor (ER)-positive and/or PR-positive] received adjuvant endocrine therapy for five years; premenopausal patients tamoxifen 20 mg/day and postmenopausal women anastrozole 1 mg daily [Citation9].
Recording of adverse events and follow-up
Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 (http://ctep.cancer.gov) [Citation14]. Left ventricle ejection fractions (LVEF) were followed up with either echocardiography or isotope cardiography according to the institutional practice during trastuzumab administration, usually at approximately three month intervals. The LVEF results were not recorded on the study database. Study participants were scheduled to be followed up for ≥ 5 years after randomization [Citation9].
Statistical analysis
Efficacy analyses were done with the intention- to-treat principle. Frequency tables were analyzed with the χ2-test or Fisher's exact test. Non-normal distributions were compared with Mann-Whitney's test or Kruskal-Wallis's analysis of variance. Survival between groups was compared using the Kaplan-Meier life-table method and the log-rank test, the hazard ratios were computed using an unadjusted Cox proportional hazards model. The subgroup analyses were performed by including the treatment group, the subgroup variable and their interaction in the Cox model. All p-values are two-sided and not adjusted for multiple testing. Statistical analyses were done with SAS® version 8.2 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Patient and tumor characteristics
Of the 284 patients with HER2-positive disease, 146 were assigned to TX-CEX and 138 to T-CEF. The characteristics of the patients and the tumors are provided in . HER2-positive tumors were more often large, ductal, poorly differentiated and steroid hormone receptor-negative as compared with HER2-negative tumors (n = 1211), and a larger proportion of the patients with HER2-positive cancer had node-negative disease and did not receive adjuvant hormonal therapy. Except for tumor histological type, the patient and tumor characteristics were similar between the subgroups of patients with HER2-positive disease who received (n = 176) or did not receive (n = 108) adjuvant trastuzumab (each p is > 0.05, ).
Table I. Patient and tumor characteristics (intention-to-treat population).
Efficacy
The median follow-up time of the 284 patients with HER2-positive disease who were alive at the time of the data lock (15 June 2012) was 6.7 years (range 0.8–8.4 years). By this date, 52 (18.3%) of the 284 patients had a RFS event and 39 (13.7%) had died. The five-year RFS of the 176 patients with HER2-positive breast cancer treated with adjuvant trastuzumab was 89.2% as compared with 75.9% in the subset of 108 patients who did not receive trastuzumab (log-rank p = 0.001; HR 0.41, 95% CI 0.23–0.72; ). Of the patients treated with trastuzumab, 92.0% were alive at five years after the date of randomization and 88.2% seven years after randomization as compared with 88.9% and 82.3% of those treated without trastuzumab, respectively (p = 0.266; HR 0.70, 95% CI 0.37–1.32).
Figure 2. Recurrence-free survival (RFS) since the date of randomization of patients with HER2-positive breast cancer. Upper panel: RFS by treatment with adjuvant trastuzumab; Lower panel: RFS by the duration of adjuvant trastuzumab. The five-year survival figures are shown. Patients who were censored are indicated with a bar.
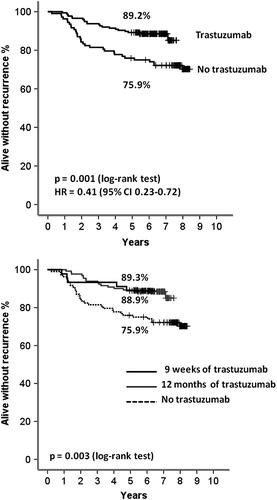
Patients who were treated for 12 months with adjuvant trastuzumab (n = 131) and those treated for nine weeks concomitantly with chemotherapy (n = 45) had similar five-year RFS (89.3% and 88.9%, respectively; p = 0.976, HR 0.98, 95% CI 0.36–2.71; ). The patient and breast cancer characteristics were not statistically different between these two groups except for cancer steroid hormone receptor expression, which was less frequent in the subset treated for nine weeks with trastuzumab (). Patients treated with nine weeks of trastuzumab received less often adjuvant hormonal therapy (due to a larger proportion of hormone receptor-negative tumors in this group) and post-operative radiotherapy. Overall survival did not differ between patients treated for 12 months or for nine weeks with trastuzumab (92.4% vs. 91.1% survived for five years, respectively; p = 0.996; HR 1.00, 95% CI 0.32–3.09).
Table II. Characteristics of patients and tumors treated with different durations of adjuvant trastuzumab.
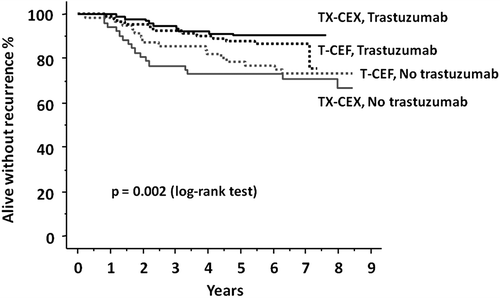
There was no interaction between adjuvant trastuzumab and the type of chemotherapy given (p = 0.248 for RFS and 0.132 for overall survival). Patients with HER2-positive disease had better RFS when treated with trastuzumab regardless of the type of chemotherapy (T-CEF or TX-CEX, ).
Figure 3. Recurrence-free survival of patients with HER2-positive breast cancer treated with docetaxel (T) followed by cyclophosphamide, epirubicin and fluorouracil (CEF) or with docetaxel and capecitabine (TX) followed by cyclophosphamide, epirubicin and capecitabine (CEX) with or without trastuzumab.
Since patients with HER2-positive disease had improved RFS after the year 2005, we estimated whether other factors than trastuzumab might have contributed to the improved RFS by comparing the outcome of the 1211 patients with HER2-negative cancer entered to the trial before and after 27 May 2005, whose study treatments remained unaltered throughout the trial accrual period. Patients with HER2-negative cancer who were randomized before or on 27 May 2005 (n = 493) turned out to have similar RFS as those randomized after 27 May 2005 (five-year RFS 85.0% vs. 86.3%, respectively; p = 0.857; HR 0.97, 95% CI 0.72–1.31), and their overall survival was also similar (five-year survival 90.9% vs. 92.1%, respectively; p = 0.828; HR 1.04, 95% CI 0.71–1.54).
Patients with HER2-positive cancer treated with adjuvant trastuzumab tended to have better RFS than women who had HER2-negative breast cancer (five-year RFS 89.2% vs. 85.8%; p = 0.084; HR 0.67, 95% CI 0.42–1.06), whereas patients with HER2-negative disease had more favorable RFS compared to the women who had HER2-positive breast cancer and did not receive trastuzumab (five-year RFS 85.8% vs. 75.9%; p = 0.007; HR 0.58, 95% CI 0.39–0.88).
Cardiac events
Six (2.1%) of the 284 patients with HER2-positive cancer and four (2.3%) of the 176 patients treated with trastuzumab were diagnosed with cardiac insufficiency or left ventricular dysfunction during the follow-up. All of these four patients treated with adjuvant trastuzumab had left ventricular dysfunction diagnosed within 13 months from the date of initiation of trastuzumab. Three of the four patients received trastuzumab for 12 months and one for nine weeks, and three were assigned to receive capecitabine. None of the patients treated with trastuzumab was diagnosed with myocardial infarction during the follow-up. The adverse effects related to the chemotherapy regimens have been reported earlier [Citation9,Citation14].
Discussion
We found in this preplanned exploratory analysis of the FinXX trial that patients with HER2-positive breast cancer who were treated with trastuzumab had better RFS than those who did not receive trastuzumab. Adjuvant trastuzumab was not administered based on random allocation in the FinXX trial. However, except for allowing adjuvant trastuzumab in the amended study protocol in 2005, the study treatments were otherwise kept unaltered, the participating study centers remained the same throughout the study, and the data are not confounded by cross-over or administration of adjuvant trastuzumab after the protocol-defined treatments had been completed. These factors generated an opportunity to investigate the effects of adjuvant trastuzumab on outcome in the setting of the FinXX trial.
The substantial improvement in RFS observed is likely due to trastuzumab administration, since there was little difference in the characteristics of the patients or the cancers treated with or without trastuzumab, and RFS of patients with HER2-negative disease who entered the study before or after amending the study protocol in May 2005 did not change with time. The current results thus provide further evidence that adjuvant trastuzumab improves RFS of women with HER2-positive early breast cancer.
There was no significant interaction between administration of adjuvant trastuzumab and the type of chemotherapy, and trastuzumab improved RFS significantly in the subset of patients treated with TX-CEX. Although capecitabine is being used extensively in the treatment of advanced breast cancer [Citation15], we are unaware of any other study that has addressed the efficacy of adjuvant trastuzumab in combination with capecitabine. It is currently controversial whether integration of capecitabine into standard adjuvant chemotherapy regimens improves outcome, and several studies addressing adjuvant capecitabine are ongoing [www.ClinicalTrials.gov]. In the U.S. Oncology Group randomized trial USON01062, where 2611 women were assigned to receive first four cycles of doxorubicin and cyclophosphamide (AC) followed by either four cycles of docetaxel or four cycles of TX, there was no significant difference in the primary endpoint (disease-free survival) between the two arms, but women treated with TX had significantly better overall survival [Citation16]. A joint analysis of the FinXX and the USON01062 trials found addition of capecitabine to a taxane-anthracycline regimen to improve significantly disease-free survival, overall survival and to reduce death from breast cancer [Citation17], whereas a randomized single-center trial that compared weekly paclitaxel with TX as adjuvant or neo-adjuvant treatments of early breast cancer was interrupted early due to absence of benefit associated with TX [Citation18].
The influence of adjuvant trastuzumab on overall survival was inconclusive. The HR of 0.68 for overall survival favored trastuzumab and is of similar magnitude as those reported from randomized adjuvant trials [Citation1–4]. Yet, as in some randomized trials addressing adjuvant trastuzumab [Citation2,Citation3,Citation19], the analysis of overall survival was not statistically significant. This might be due to the limited numbers of deaths encountered, cross-over between arms [Citation2] or the treatments given for advanced breast cancer, which may influence the overall survival rates [Citation20,Citation21].
Although the standard duration of adjuvant trastuzumab administration is currently 12 months, the optimal duration remains unknown. The recent findings from the HERA trial suggest that two years of trastuzumab administration may be regarded as inferior to one year of administration, since two years of trastuzumab does not improve survival but results in more cardiac toxicity [Citation22] and requires more healthcare resources [Citation23,Citation24]. Results from the PHARE trial that compares six versus 12 months of adjuvant trastuzumab remained inconclusive after a relatively short median follow-up time of 3.5 years [Citation25]. The present results on nine-week administration of trastuzumab concomitantly with docetaxel provide support for continuation of the ongoing randomized adjuvant trials that evaluate short durations of trastuzumab, since we found no difference in RFS or survival between the subsets of patients who received trastuzumab for nine weeks or for 12 months. The characteristics of the patients and tumors were similar in the groups except for presence of more hormone receptor-negative cancers in the 9-week group. This difference, which likely occurred by chance, is unlikely to favor the 9-week group [Citation26]. This observation should, however, be interpreted with caution due to the small number of patients who were treated with 9-week duration of trastuzumab (n = 45), the retrospective and exploratory nature of the analysis, and administration of single-agent trastuzumab after completion of chemotherapy to most patients in the 12-month group, which is probably less effective as compared with concomitant administration [Citation4].
The results indicate that women diagnosed with HER2-positive early breast cancer treated with trastuzumab generally have favorable outcome. The seven-year RFS was as high as 89.2% despite we included only patients with a moderate or high risk of breast cancer recurrence in the FinXX trial. Of note, RFS of these women tended to be even better than that of the patients with HER2-negative disease. The study accrued a large number of patients from a relatively small population and a short time period, which may reduce the risk of a major selection bias.
In conclusion, the observations from this planned exploratory analysis of a randomized FinXX trial suggest that adjuvant trastuzumab improves RFS of patients treated with adjuvant T-CEF or TX-CEX chemotherapy, and that trastuzumab is safe and effective when administered to patients who receive an adjuvant regimen that contains capecitabine integrated with standard agents, such as TX-CEX. The findings support continuation of the testing of regimens where trastuzumab is administered concomitantly with chemotherapy for a short time period in controlled randomized trials. RFS of patients with moderate- or high-risk HER2-positive breast cancer treated with adjuvant trastuzumab has turned favorable, and may now be better than that of patients with moderate- or high-risk HER2-negative breast cancer.
Declaration of interest: The authors alone are responsible for the content and writing of the paper.
The FinXX study was sponsored by the Finnish Breast Cancer Group, and supported financially by Roche, Sanofi-Aventis, AstraZeneca, Cancer Society of Finland, Academy of Finland, Helsinki University Research Funds, Sigrid Juselius Foundation. We are indebted to the study monitor Ms. Raija Husa, and many medical, nursing, and clerical staff members for the support at the participating centers; and to all women participating in the FinXX trial for their contribution to this research. The clinical research institute of HJ has received research funding from Sanofi and Novartis; P-LK-L has received honoraria, research funding and other remuneration from Sanofi, Roche and Pfizer; MT honoraria or remuneration from AstraZeneca, GlaxoSmithKline, Roche, MSD and Novartis; LH a fee from Roche; GN honoraria from Roche, Sanofi and AstraZeneca; and VK honoraria from Roche and Sanofi. RH, AJ-V, RK, JA, PA, OS, KV, PN, ML, PB and HL declare no conflict of interest.
References
- Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE Jr, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 2011;29:3366–73.
- Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirch A, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: A 4-year follow-up of a randomised controlled trial. Lancet Oncol 2011;12:236–44.
- Joensuu H, Kellokumpu-Lehtinen P-L, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006;354:809–20.
- Perez EA, Suman VJ, Davidson NE, Gralow JR, Kaufman PA, Visscher DW, et al. Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol 2011;29:4491–7.
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273–83.
- Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012;4: CD006243.
- Perez EA. Cardiac toxicity of ErbB2-targeted therapies: What do we know?Clin Breast Cancer 2008;8(Suppl 3):S114–20.
- Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Goss P. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol 2012;60:2504–12.
- Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, Jukkola-Vuorinen A, Tanner M, Kokko R, et al. Adjuvant capecitabine, docetaxel, cyclophosphamide, and epirubicin for early breast cancer: Final analysis of the randomized FinXX trial. J Clin Oncol 2012;30:11–8.
- Liu S, Chia SK, Mehl E, Leung S, Rajput A, Cheang MC, et al. Progesterone receptor is a significant factor associated with clinical outcomes and effect of adjuvant tamoxifen therapy in breast cancer patients. Breast Cancer Res Treat 2010;119:53–61.
- Rydén L, Haglund M, Bendahl PO, Hatschek T, Kolaric A, Kovács A, et al. Swedish Her2 Analysis Group. Reproducibility of human epidermal growth factor receptor 2 analysis in primary breast cancer: A national survey performed at pathology departments in Sweden. Acta Oncol 2009;48: 860–6.
- Piccart-Gebhart M, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659–72.
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;53:1673–84.
- Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, Jukkola-Vuorinen A, Tanner M, Asola R, et al. Adjuvant capecitabine in combination with docetaxel and cyclophosphamide plus epirubicin for breast cancer: An open-label, randomised controlled trial. Lancet Oncol 2009;10:1145–51.
- Pallis AG, Boukovinas I, Ardavanis A, Varthalitis I, Malamos N, Georgoulias V, et al. A multicenter randomized phase III trial of vinorelbine/gemcitabine doublet versus capecitabine monotherapy in anthracycline- and taxane-pretreated women with metastatic breast cancer. Ann Oncol 2012;23:1164–9.
- O´Shaughnessy J, Stokoe PD, Pippen Jr J, Blum JL, Krekow L, Holmes FA, et al. First efficacy results of a randomized, open-label, phase III study of adjuvant doxorubicin plus cyclophosphamide, followed by docetaxel with or without capecitabine, in high-risk early breast cancer. Cancer Res 2010;70:85s (abstract).
- Jiang Y, Yin W, Zhou L, Yan T, Zhou Q, Du Y, et al. First efficacy results of capecitabine with anthracycline- and taxane-based adjuvant therapy in high-risk early breast cancer: A meta-analysis. PLoS One 2012;7:e32474.
- Kelly CM, Green MC, Broglio K, Thomas ES, Brewster AM, Valero V, et al. Phase III trial evaluating weekly paclitaxel versus docetaxel in combination with capecitabine in operable breast cancer. J Clin Oncol 2012;30:930–5.
- Spielmann M, Roché H, Delozier T, Canon JL, Romieu G, Bourgeois H, et al. Trastuzumab for patients with axillary node-positive breast cancer: Results of the FNCLCC-PACS 04 trial. J Clin Oncol 2009;27:6129–34.
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92.
- Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat 2008;112:533–43.
- Goldhirsch A, Piccart-Gebhart MJ, Procter M, de Azambuja E, Weber HA, Untch M, et al. HERA TRIAL: 2 years versus 1 year of trastuzumab after adjuvant chemotherapy in women with early HER2-positive breast cancer at 8 years of median follow-up. Cancer Res 2012;72(24 Suppl):103S–4S (abstract).
- Purmonen TT, Pänkäläinen E, Turunen JH, Asseburg C, Martikainen JA. Short-course adjuvant trastuzumab therapy in early stage breast cancer in Finland: Cost-effectiveness and value of information analysis based on the 5-year follow-up results of the FinHer Trial. Acta Oncol 2011;50: 344–52.
- Lidgren M, Wilking N, Jönsson B, Rehnberg C. Cost- effectiveness of HER2 testing and trastuzumab therapy for metastatic breast cancer. Acta Oncol 2008;47:1018–28.
- Pivot X, Romieu G, Bonnefoi H, Pierga J-Y, Kerbrat P, Guastalla J-P, et al. PHARE Trial results of subset analysis comparing 6 to 12 months of trastuzumab in adjuvant early breast cancer. Cancer Res 2012;72(24 Suppl.):104S (abstract).
- Untch M, Untch M, Gelber RD, Jackisch C, Procter M, Baselga J, et al. Estimating the magnitude of trastuzumab effects within patient subgroups in the HERA trial. Ann Oncol 2008;19:1090–6.