Abstract
Background. How to assess cachexia is a barrier both in research and in clinical practice. This study examines the need for assessing both reduced food intake and loss of appetite, to see if these variables can be used interchangeably. A secondary aim is to assess the variance explained by food intake, appetite and weight loss by using tumor-related factors, symptoms and biological markers as explanatory variables. Material and methods. One thousand and seventy patients with incurable cancer were registered in an observational, cross sectional multicenter study. A total of 885 patients that had complete data on food intake (PG-SGA), appetite (EORTC QLQ-C30) and weight loss were included in the present analysis. The association between reduced food intake and appetite loss was assessed using Spearman's correlation. To find the explained variance of the three symptoms a multivariate analysis was performed. Results. The mean age was 62 years with a mean survival of 247 days and a mean Karnofsky performance status of 72. Thirteen percent of the patients who reported eating less than normal had good appetite and 25% who had unchanged or increased food intake had reduced appetite. Correlation between appetite loss and food intake was 0.50. Explained variance for the regression models was 44% for appetite loss, 27% for food intake and only 13% for weight loss. Conclusion. Both appetite loss and food intake should be assessed in cachectic patients since conscious control of eating may sometimes overcome appetite loss. The low explained variance for weight loss is probably caused by the need for more knowledge about metabolism and inflammation, and is consistent with the cancer cachexia definition that claims that in cachexia weight loss is not caused by reduced food intake alone. The questions concerning appetite loss from EORTC-QLQ C30 and food intake from PG-SGA seem practical and informative when dealing with advanced cancer patients.
Patients with advanced, incurable cancer frequently experience involuntary weight loss. Weight loss in cancer is usually a consequence of either reduced nutritional intake or uptake of nutrients, or of cancer cachexia. Reduced nutritional intake or uptake is defined as starvation, and will normally respond well to nutritional treatment. Cancer cachexia is caused by a combination of decreased nutritional intake and altered metabolism, and is defined as ongoing loss of skeletal muscle mass that cannot be reversed by conventional nutritional support [Citation1]. It is characterized by progressive functional impairment [Citation1] and contributes to more than 20% of cancer deaths [Citation2]. Since around 50% of patients with malignant disease cannot be cured and more than 80% of advanced cancer patients experience cachexia [Citation2], the toll of this condition is severe both for society and the individual patient.
An international cachexia consensus indicated four domains to be assessed in cachexia: 1) catabolism driven by inflammation, tumor products, and neuro-hormonal alterations including hypo-anabolism; 2) anorexia or reduced food intake; 3) loss of muscle mass and a variable loss of fat mass; and 4) reduced physical/psychosocial function, e.g. manifesting as physical fatigue and psychosocial distress [Citation1]. There is a wide range of items that may be of interest when measuring these domains, i.e. various biomarkers, patient-reported outcomes or several disease-related patient characteristics. The consensus offered little guidance on which items to use, but for the nutrition domain it was recommended that patients’ own estimates of total food intake compared to normal should be registered, as well as probable causes for reduced intake.
Cancer cachexia was previously referred to as the cancer cachexia-anorexia syndrome, as anorexia is frequently encountered. There is, however, no agreement about how to diagnose anorexia, and different instruments (e.g. AQ, VAS, AC/S-12, FAACT) all measure different aspects of the condition [Citation3]. Anorexia can be regarded as an overarching concept consisting of a variety of symptoms such as appetite loss, early satiety, taste alterations [Citation4], reduced food intake, meat aversions and nausea/vomiting [Citation5]. In daily practice, appetite and anorexia are commonly used as synonyms as both can be defined as loss of desire to eat [Citation3,Citation5]. In the following this definition of anorexia and appetite loss will be applied.
The term “appetite loss” can be perceived as an indicator of both quality of life and cancer severity, and is independently linked to survival [Citation6] and correlates significantly with physical function [Citation7]. The pathophysiology behind primary appetite loss in advanced cancer is probably in part caused by pro-inflammatory cytokines and neuro-hormonal alterations [Citation8], and seems accordingly to be related to the host reaction to tumor. Appetite loss may, however, also be affected by secondary factors such as depression/psychosocial stress, nausea, constipation, taste alterations or pain [Citation6,Citation9].
The item “food intake” is logically associated with appetite loss or factors causing appetite loss, but can also be influenced by a wide range of factors like cognitive impairment [Citation10], vomiting, stomatitis, dysphagia, dietary restrictions etc. [Citation11].
The lack of knowledge and agreement about how to assess weight loss and cachexia has been an important barrier in the interpretation of cachexia research and in the clinical treatment of involuntary weight loss. It is therefore important to work towards a common functional understanding of which items to consider and which to bypass in the evaluation of patients with advanced cancer and weight loss ().
A systematic review of items measured in clinical studies of weight loss showed that dietary records were sporadically reported, that many studies only extrapolated the amount of food intake from self- reported appetite, and that appetite loss and energy intake were inconsistently related to weight loss [Citation12].
There are indications that reduced food intake and appetite loss are distinct items [Citation13]. There is, however, still controversy concerning the necessity to assess both self-reported food intake and appetite as a part of the nutritional domain in cachexia, or if these measurements can be used interchangeably. There is also insufficient information as to which factors are associated with food intake, appetite loss and weight loss in advanced cancer.
Our primary aim was to examine the association between reduced food intake and appetite loss to confirm our hypothesis of a modest correlation. The secondary aim was to evaluate the associations between food intake, appetite loss and weight loss with tumor-related factors, symptoms, survival and CRP. The third objective was to examine whether reduced food intake, appetite loss and weight loss can be explained by symptom burden and treatment- or disease-related factors.
Material and methods
Patients and study design
Between October 2008 and December 2009, 1070 patients were registered in the European Palliative Research Collaborative – Computerized symptom assessment study (EPCRC-CSA) [Citation14]. This observational, cross sectional multicenter study aimed to advance the development of a computer-based symptom assessment and classification tool for pain, cachexia and depression. A major research objective was to characterize the different symptoms, and investigate how these should be assessed. The choice of items was based on the work of the EPCRC. The assessment and classification tool is intended for use in clinic and research. Adult patients with incurable cancer and metastatic or advanced disease were recruited from 17 centers in Norway, UK, Austria, Germany, Switzerland, Italy, Canada and Australia [Citation14].
Data collection
The entire data collection was performed using touch-sensitive computers (HP Compaq TC4200 1200 tablet PCs). Specific details regarding methods and instruments have been presented elsewhere [Citation14]. One part of the registration was completed by healthcare professionals, the other by the patients. A research nurse assisted patients if necessary. Healthcare professionals obtained and recorded information on age, gender, height, weight, blood tests and treatment. Cancer diagnosis including stage of disease and metastases with localization was also recorded.
Patients’ subjective health was measured by the European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire, the EORTC QLQ-C30 version 3.0 [Citation15]. This well- validated and extensively used 30-item tool is specifically developed for cancer patients [Citation16]. The items form five functional scales, three symptom scales and six single items. Appetite loss during the last week is measured on a four-point categorical scale (not at all – very much).
Three of four items from the Patient-Generated Subjective Global Assessment (PG-SGA) [Citation17] were used to assess weight loss and food intake: patient-reported current weight, weight change in the last six months, and changed food intake compared to normal on a three-point categorical scale (less than usual – more than usual).
Since cachexia was not the primary study outcome and it is essential not to overburden the patients, other relevant items for cancer cachexia such as a checklist for nutrition-impact symptoms, tests for physical function, presence of clinically relevant edema or registrations of tumor activity were not part of the EPCRC-CSA assessment. The last retrospective registration of death was completed by 31 December 2010.
Statistical considerations
For all analyses, “weight loss” was defined as self-reported weight loss the last six months expressed as a percentage. Patients were assumed to have reduced food intake if they rated their food intake as less than usual compared to normal (both items from PG-SGA). Patients were considered to have reduced appetite if they reported a little, quite a bit or very much appetite loss on the EORTC QLQ-C30 questionnaire.
The analytical approach in this paper consists of three steps.
In the first step, Spearman's correlation was calculated to explore the relationship between self- reported reduced food intake and appetite loss. The next two analytical steps examined whether food intake, appetite and weight loss are distinctive assessments.
In the second step, univariate analyses were performed to describe associations between tumor- related factors, symptoms and biological markers and levels of food intake, appetite and weight loss. The factors explored were all 15 scales from EORTC QLQ-C30, weight loss, food intake, origin of cancer, metastasis site, treatment and CRP. CRP was log normally distributed and was transformed before the regression analysis.
The impact on survival of food intake, weight loss and appetite loss was explored using univariate log-rank tests. Significant factors (p < 0.05) were included in a multivariate survival analysis using Cox-model regression.
In the third step, multivariate regression analyses were conducted to determine the portion of variance explained by weight loss, appetite loss and reduced food intake. All factors used in the univariate analysis in step two were regarded as explanatory variables. Factors were then explored for associations using a mixed forward-backwards stepwise multivariate regression analysis and included if significant (p < 0.05). Factors were reported if the standardized regression coefficients were larger than 0.1. The aim was to find the factors that best predicted appetite, food intake and weight loss. A multiple regression analysis such as this should be regarded as exploratory, as it will not provide a definitive set or of variables that contribute to the three symptoms since these will vary with the studied population and the strength of connections between the factors evaluated.
Results
Patient demographics
A total of 885 patients with complete data on weight loss, appetite loss and food intake were available for analysis after excluding four patients due to withdrawal of informed consent, 15 patients because of technical failure, and 166 patients with incomplete data on food intake (n = 82), appetite loss (n = 37) or weight loss (n = 166).
Mean age was 62 years (range 18–89) and 53.4% were male patients. Most patients (n = 535) had metastatic tumors originating from digestive organs, breast or lung. At inclusion, 254 patients were not receiving active tumor treatment. The mean survival was 247 days (95% CI 233–261) and mean Karnofsky performance status was 72 (range 20–100). The charecteristics of the patients are reported in .
Table I. Demographic data.
Correlation between weight loss, appetite loss and change in nutritional intake
Eating less than normal was reported by 13% of the patients who had good appetite, while 25% of the patients who had reduced appetite ate more than usual or unchanged (). Correlation between appetite loss and food intake was 0.50. The correlations between weight loss and food intake, and weight loss and appetite were both 0.34.
Table II. Comparison of appetite loss and food intake.
Distribution of symptoms, tumor-related factors, biological markers and survival
Neither food intake nor appetite loss was significantly associated with tumor or site of metastases in the univariate analysis. Reduced food intake was associated with higher age. Weight loss was associated with gender (higher if male), and had a weak negative correlation with tumors originating from the breast as these patients had significantly less weight loss compared to the rest of the population (2.2% vs. 5.2%). Weight loss, appetite and food intake were associated with the current use of chemotherapy, with patients treated with chemotherapy having significantly less weight loss (3.4% vs. 5.7%), describing their appetite as better and their food intake as closer to normal. All three symptoms were associated with the use of anti-emetics (other than glucocorticoids), more nausea/vomiting and increased CRP-values. All variables from EORTC QLQ-C30 were associated with appetite loss. All EORTC QLQ-C30 variables except insomnia, diarrhea and financial situation were associated with reduced food intake and weight loss.
The standardized βs imply that, for all three symptoms, age, gender, tumor localization, metastatic site and current therapy had less impact than most variables on the EORTC QLQ-C30 scale and LogCRP ().
Table III. Univariate linear regression.
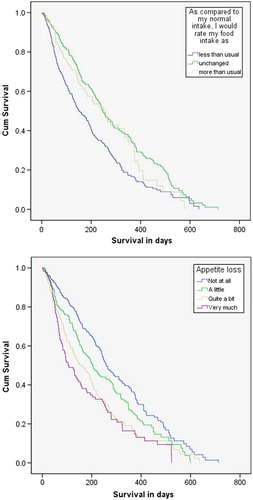
In univariate analysis food intake, appetite and weight loss were significantly associated with reduced survival (). In the multivariate survival analysis only weight loss (p = 0.01) and appetite loss (p < 0.001) remained significantly associated with reduced survival.
Multiple regression analysis
To determine the need for registering additional information in future studies, a multiple regression analysis was performed to examine how well weight loss, appetite and food intake could be explained by other factors (). Explained variance for the regression models (adjusted R2) was 44% for appetite loss, 27% for food intake and 13% for weight loss. Explanatory variables used were demographic, tumor-related and treatments factors as well as CRP, self-reported quality of life, nutritional intake and weight loss.
Table IV. Multiple regression analysis*.
Appetite loss, LogCRP and weight loss were significantly associated with food intake, but appetite had the highest standardized β (0.42 compared to 0.11 and 0.13). Food intake, fatigue and nausea/vomiting were significantly associated with appetite loss, with similar standardized βs (0.33, 0.26 and 0.25, respectively). Physical function, appetite loss, food intake and gender were significantly associated with weight loss and had similar standardized βs (0.15, 0.15, 0.16 and 0.11).
Discussion
In this observational, cross sectional, multicenter study, we explored whether food intake and appetite could be used as equivalent measurements in the assessment of cachexia in advanced cancer patients. In the studied population the correlation between food intake and appetite loss was r = 0.50 (p < 0.01), a rather moderate association. In a substantial number of patients (25%) self-reported food intake increased or remained unchanged in spite of some degree of appetite loss, and 13% ate less despite reporting good appetite. These data are consistent with the primary hypothesis, suggesting that the two factors, food intake and appetite loss, cannot be used interchangeably. This means that they cannot be substituted for one another or be extrapolated from each other when looking into the complexity of weight loss in e.g. cancer cachexia.
In order to understand how food intake, appetite and weight loss might be interrelated, regression analysis was performed with these items as explanatory variables. In this population other symptoms showed a higher association with food intake and appetite loss than variables related to the cancer disease and treatment in both univariate and multivariate analysis.
The multivariate analysis showed that dissimilar variables were associated with food intake, appetite and weight loss, and that it was possible to explain 44% of the variance of appetite loss, 27% of the variance of food intake, but only 13% of the variance of weight loss. This suggests that there might be differences as to which factors best predict appetite loss, food intake and weight loss, and also that the present variables are insufficient to explain these symptoms. Therefore additional factors such as other nutritional impact symptoms (early satiety, mouth sores, dysphagia, and information on metabolism) should be assessed. A longitudinal study of newly diagnosed head and neck cancer patients found that symptoms such as pre-treatment anorexia, pain, dysphagia and mouth sores were predictors of reduced dietary intake and weight [Citation11].
It was possible to explain more of appetite loss and reduced food intake than weight loss in the multivariate analysis. Furthermore, the correlations of weight loss to appetite loss and to reduced food intake were low to moderate (both r = 0.34, p < 0.01). This is probably because of a gap in the understanding of metabolism and inflammation in relation to weight loss; consistent with the cancer cachexia definition that claims that cachexia/weight loss is not caused by reduced food intake alone. CRP was associated with weight loss in the univariate analysis, but not in the multivariate analysis. A review of items associated with weight loss found that albumin, CRP and IL-6 were the inflammation markers most consistently associated with weight loss [Citation12]. Results for other cytokines such as TNFα, IL-10, IL-8, IL-4, IFN-γ were more inconsistent or mainly not significant [Citation12]. Nevertheless, CRP is today probably the most robust biomarker for cachexia [Citation18], although it is highly unspecific and new biomarkers are warranted. It is also important to acknowledge that cachexia can occur without manifest systemic inflammation [Citation1].
In the current study it is evident that advanced cancer patients with appetite loss have a greater symptom burden and reduced survival compared to patients with weight loss alone. If assessing cachectic patients without considering appetite loss, one would lose an important predictor of survival, an important aspect of quality of life as well as an important topic for information/psychosocial intervention [Citation19]. Information from the present study supports the suggestions that “cachexia with anorexia” should be a sub-type of cachexia [Citation4] or a part of a grading system for cachexia [Citation1,Citation20]. While arguing for this, some emphasize that appetite loss and loss of weight/muscle have similarities in their pathophysiology as they both might be affected by inflammation [Citation21], while others propose a more diverse pathophysiology [Citation4].
The present results are based on selected variables, which represent some but not all possible items in the four cachexia domains. Body weight was available, but no information on edema or direct measures of muscle mass was assessable, which might reduce the value of weight loss as an indicator. In the analyses we investigated self-reported weight and weight loss, the correlation between self-reported weight and caregiver reported weight was in this study r = 0.97 (numbers not shown).
For the nutritional domain there was no information on early satiety or symptoms such as xerostomia, stomatitis or changes in sense of smell, but the measures for appetite seem robust. For the catabolism domain, there was no information on current cancer disease dynamics (e.g. whether the cancer was resistant to anticancer treatment and progressing) or hypo-anabolism, but the dataset contains the important marker CRP. Considering the functional domain, the dataset contains self-reported physical function, but no objective measures of muscle strength. It is not possible to evaluate whether the inclusion of these additional variables would have changed the present results substantially.
This was a cross sectional study and there were no documented measurements of what the patients used to eat or what they actually were eating at inclusion. Only patients’ own estimate of food intake in relation to normal intake was reported in accordance with the minimal requirements described in the international consensus [Citation1]. This assessment of food intake has however not been compared with precise longitudinal measurements of nutritional intake, and as mentioned self-reported estimates are known to have some bias [Citation22]. The self-reported question of food intake has not been validated against prospectively collected diet records and there is a possibility that patients have been eating less than they report; in this case the correlation between weight loss and food intake might have been higher if the information on food intake were based on precise measurements of food intake instead of self-reported information.
This study nevertheless demonstrates that food intake needs to be assessed in addition to appetite loss. Even though cachexia cannot be cured with nutrition alone [Citation1], it is important to secure sufficient energy and protein intake and avoid under-nutrition in patients with curative cancer or with pre-cachexia/cachexia. Consultations with nutritional professionals (registered dietitian or equivalent) might be of great value [Citation23]. In patients with late cachexia overview on food intake is essential to enable patient information. In this situation nutritional advice should be given in a manner that attempts to relieve the burden for patient and their care givers. One example of this might be to end the focus on calories and help increase meal enjoyment by lowering demands and expectations.
In this population, grading of appetite loss on a four-point scale gave a more nuanced prediction of survival and returned higher significance levels than the question of reduced intake. One reason for this might be that some patients who realize they have short expected survival might eat more in the hope of fighting heir cancer disease. Another possibility is that patients might claim to eat more, even though they eat less or the same, in order to give the clinician the supposedly “right answer”. The focus on eating enough and healthily might also be a reason for the moderate correlation between appetite and food intake. Patients who know they are at risk of losing weight may force themselves to eat despite lack of appetite. Thus, treating loss of appetite might not affect the intake in some patients but might affect their feelings and joy of their meals.
The cross sectional design can only evaluate associations, not causality. Weight loss in cachexia is known to be caused not only by reduced food intake but also by additional factors; appetite loss and weight loss might be concurrent events caused in part by common pathophysiology. Reduced food intake is, however, likely to accelerate weight loss. The present study design does not give information on consequences for weight, quality of life variables and survival following improved nutrition or appetite.
A problem when studying these issues is to untangle the question of whether appetite loss and reduced food intake are merely irreparable indicators of patients in whom treatment soon will fail, or whether the symptoms decrease the ability of some patients to be treated and that modifications can lead to improved survival or quality of life. Large prospective studies with well-defined assessments and management of both malnutrition and cachexia are needed in order to answer this question.
Information in this paper is based on a large multicenter study with advanced cancer of heterogeneous origin. It may contribute to the ongoing work on assessment of cancer cachexia, which builds on the cancer cachexia framework. Currently it is difficult to differentiate well between starvation/malnutrition and cachexia, partly because these conditions are intertwined in varying degrees. Hopefully, the future refinement of the cachexia classification system will help discriminate somewhat between these conditions, and thus improve treatment strategies. The current cross-sectional study did not define cachexia as an inclusion criterion and therefore a mixture of patients with and without malnutrition and cachexia, were included which provides an opportunity to describe the comprehensive cancer population well.
Conclusion
The intention of the present study was to contribute to the clinical understanding of which items are necessary for the assessment of a cachectic patient, and by this to the progression of the international classification system for cachexia.
Cachexia is defined by weight loss and is characterized by several domains. Both appetite loss and food intake should be assessed in the characterization of cachexia as each of these symptoms appears to provide distinct information. One of the reasons for this is that anorexia can sometimes be overcome by conscious control of eating [Citation24]. Patients who know they are sick and have a flawed appetite regulation may force themselves to eat without appetite.
The questions concerning appetite loss from EORTC-QLQ C30 and food intake from PG-SGA are practical and seem informative when assessing advanced cancer patients.
Acknowledgement
The EPCRC (2006–2010) was funded by the European Commission’s Sixth Framework Programme (contract no LSHC-CT-2006–037777) with the overall aim to improve treatment of pain, depression, and fatigue through translation research. Core scientific group/work package leaders: Stein Kaasa (project coordinator), Frank Skorpen, Marianne Jensen Hjermstad, and Jon Håvard Loge, Norwegian University of Science and Technology; Geoffrey Hanks, University of Bristol; Augusto Caraceni and Franco De Conno, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan; Irene Higginson, King’s College London; Florian Strasser, Cantonal Hospital St. Gallen; Lukas Radbruch, RWTH Aachen University; Kenneth Fearon, University of Edinburgh; Hellmut Samonigg, Medical University of Graz; Ketil Bø, Trollhetta AS, Norway; Irene Rech-Weichselbraun, Bender MedSystems GmbH, Austria; Odd Erik Gundersen, Verdande Technology AS, Norway. Scientific advisory group: Neil Aaronson, The Netherlands Cancer Institute; Vickie Baracos and Robin L. Fainsinger, University of Alberta; Patrick C. Stone, St. George’s University of London; Mari Lloyd- Williams, University of Liverpool. Project management: Stein Kaasa, Ola Dale, and Dagny F. Haugen, Norwegian University of Science and Technology. There are no financial benefits or conflicts of interest that might bias this work.
Declaration of interest: The authors alone are responsible for the content and writing of the paper.
References
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 2011;12:489–95.
- Fearon KC. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur J Cancer 2008; 44:1124–32.
- Arezzo di Trifiletti A, Misino P, Giannantoni P, Giannantoni B, Cascino A, Fazi L, et al. Comparison of the performance of four different tools in diagnosing disease-associated anorexia and their relationship with nutritional, functional and clinical outcome measures in hospitalized patients. Clin Nutr 2012;20:00257–9.
- Davis MP, Dreicer R, Walsh D, Lagman R, LeGrand SB. Appetite and cancer-associated anorexia: A review. J Clin Oncol 2004;22:1510–7.
- Laviano A, Meguid MM, Rossi-Fanelli F. Cancer anorexia: Clinical implications, pathogenesis, and therapeutic strategies. Lancet Oncol 2003;4:686–94.
- Shragge JE, Wismer WV, Olson KL, Baracos VE. The management of anorexia by patients with advanced cancer: A critical review of the literature. Palliat Med 2006;20: 623–9.
- Lis CG, Gupta D, Grutsch JF. Can anorexia predict patient satisfaction with quality of life in advanced cancer?Support Care Cancer 2009;17:129–35.
- Grossberg AJ, Scarlett JM, Marks DL. Hypothalamic mechanisms in cachexia. Physiol Behav 2010;100:478–89.
- Davis MP, Dickerson D. Cachexia and anorexia: Cancer’s covert killer. Support Care Cancer 2000;8:180–7.
- Johansson L, Sidenvall B, Malmberg B, Christensson L. Who will become malnourished? A prospective study of factors associated with malnutrition in older persons living at home. J Nutr Health Aging 2009;13:855–61.
- Kubrak C, Olson K, Jha N, Jensen L, McCargar L, Seikaly H, et al. Nutrition impact symptoms: Key determinants of reduced dietary intake, weight loss, and reduced functional capacity of patients with head and neck cancer before treatment. Head Neck 2010;32:290–300.
- Blum D, Omlin A, Baracos VE, Solheim TS, Tan BH, Stone P, et al. Cancer cachexia: A systematic literature review of items and domains associated with involuntary weight loss in cancer. Crit Rev Oncol Hematol 2011;80:114–44.
- Sarhill N, Mahmoud F, Walsh D, Nelson KA, Komurcu S, Davis M, et al. Evaluation of nutritional status in advanced metastatic cancer. Support Care Cancer 2003;11:652–9.
- Hjermstad MJ, Lie HC, Caraceni A, Currow DC, Fainsinger RL, Gundersen OE, et al. Computer-based symptom assessment is feasible in patients with advanced cancer: Results from an international multicenter study, the EPCRC-CSA. J Pain Symptom Manage 2012;44:639–54.
- Aaronson NK Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Bjordal K, de Graeff A, Fayers PM, Hammerlid E, van Pottelsberghe C, Curran D, et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H & N35) in head and neck patients. EORTC Quality of Life Group. Eur J Cancer 2000;36:1796–807.
- Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status?J Parenter Enteral Nutr 1987;11:8–13.
- Tan BH, Deans DA, Skipworth RJ, Ross JA, Fearon KC. Biomarkers for cancer cachexia: Is there also a genetic component to cachexia?Support Care Cancer 2008;16:229–34.
- Hopkinson JB, Fenlon DR, Okamoto I, Wright DN, Scott I, Addington-Hall JM, et al. The deliverability, acceptability, and perceived effect of the Macmillan approach to weight loss and eating difficulties: A phase II, cluster-randomized, exploratory trial of a psychosocial intervention for weight- and eating-related distress in people with advanced cancer. J Pain Symptom Manage 2010;40:684–95.
- Bozzetti F, Mariani L. Defining and classifying cancer cachexia: A proposal by the SCRINIO Working Group. J Parenter Enteral Nutr 2009;33:361–7.
- Bennani-Baiti N, Davis MP. Cytokines and cancer anorexia cachexia syndrome. Am J Hosp Palliat Care 2008;25:407–11.
- Groenvold M, Fayers PM, Sprangers MAG, Bjorner JB, Klee MC, Aaronson NK, et al. Anxiety and depression in breast cancer patients at low risk of recurrence compared with the general population: A valid comparison?J Clin Epidemiol 1999;52:523–30.
- Ravasco P, Monteiro-Grillo I, Camilo M. Individualized nutrition intervention is of major benefit to colorectal cancer patients: Long-term follow-up of a randomized controlled trial of nutritional therapy. Am J Clin Nutr 2012;96:1346–53.
- Shragge JE, Wismer WV, Olson KL, Baracos VE. Shifting to conscious control: Psychosocial and dietary management of anorexia by patients with advanced cancer. Palliat Med 2007;21:227–33.