Abstract
Background. Dysphagia is a common and debilitating side effect in head and neck radiotherapy (RT). Prognostic factors are numerous and their interrelationship not well understood. The aim of this study was to establish a multivariate prognostic model for acute and late dysphagia after RT, based on information from a prospective trial. Material and methods. The DAHANCA 6&7 randomized study included 1476 patients with head and neck cancer eligible for primary RT alone. Patients were randomized between 5 and 6 weekly fractions of conventional RT, and received 62–70 Gy in 31–35 fractions. Patients were scored for dysphagia weekly during treatment and at regular intervals until five years after treatment. Dysphagia scores were available from 1461 patients. Results. Acute dysphagia according to DAHANCA grades 1, 2, 3 and 4 occurred in 83%, 71%, 43% and 23%, respectively. Severe dysphagia occurred in 47% and 38% of patients receiving accelerated or conventional radiotherapy, respectively (p = 0.001). At one, two, three, four and five years the prevalence of chronic dysphagia above grade 0, was 46%, 32%, 29%, 24%, 23%, respectively with no difference between 5 and 6 fractions. In multivariate analysis, the following parameters were independent factors for severe acute dysphagia: T3–T4 tumors, N-positive disease, non-glottic cancer, age> median, baseline dysphagia > 1 and accelerated radiotherapy. The following factors were prognostic factors for late dysphagia: non-glottic cancer, T3–T4, N-positive disease and baseline dysphagia > 1. The data confirmed previously published predictive models, as it was possible to separate patients in groups with low, medium and high risk of dysphagia, respectively, based on pre-treatment risk scores. Conclusion. Prognostic models were established to characterize patients at risk of developing acute or late dysphagia in the DAHANCA 6&7 trial. The results may be useful to identify patients at risk of dysphagia and thus candidates for prophylactic measures against swallowing dysfunction.
Late morbidity related to head and neck cancer treatment has become increasingly important since more cancer patients become long-term survivors. One of the predominant side effects is dysphagia [Citation1]. Swallowing is a complex coordinated process involving many critical structures and radiotherapy (RT) may cause damage to many of these swallowing structures, including sensory and motor nerves, muscles, glands, joints and connective tissue. A significant proportion of patients may consequently experience dysphagia after treatment [Citation1] or the treatment may increase the frequency, severity and clinical manifestations of disorders present before treatment [Citation2]. In order to select the patients who may benefit from prophylactic procedures like dysphagia sparing IMRT or swallowing exercises, it is essential to be able to identify patients with high risk of late dysphagia as early as possible. Knowledge about prognostic factors for the risk of developing swallowing dysfunction are growing but still not well established; they may include the extent and localization of the primary tumor, as well as patient or treatment characteristics.
The aim of this study was to establish a multivariate prognostic model for acute and late dysphagia after RT, based on information from a large prospective trial.
Material and methods
The main study set-up has been described in details previously [Citation3]. In brief, the DAHANCA 6&7 multicenter trial randomized patients to receive either 5 or 6 weekly fractions of conventional radiotherapy. A total of 1485 patients with stage I–IV squamous cell carcinoma of the oral cavity, pharynx and larynx were randomized between January 1992 and December 1999. The primary endpoint of the randomized study was locoregional control after radiotherapy, and secondary endpoints were local T site and regional N site control, voice preservation, disease-specific survival, overall survival and early and late treatment-related morbidity.
The current analysis included data on dysphagia from 1461 patients, patient and treatment characteristics are presented in . The DAHANCA morbidity scoring system for dysphagia used in this study is a five-step scale ranging from grade 0 (no dysphagia) to grade 4 (significant dysphagia even with liquid diet). Each step distinguishes between both the degree of dysphagia and the impact on daily life due to the ability to eat various consistencies of food. Dysphagia was scored prospectively by the physician weekly during treatment, two months after treatment and subsequently every three months in the first year, every fourth month in the second year followed by every six months for up to five years after randomization. Median follow-up was 4.1 years (range 0–11.5 years).
Table I. Patient and treatment characteristics.
Treatment
Patients were treated according to the DAHANCA radiotherapy guidelines as previously described [Citation3], generally using opposing lateral and shrinking fields. Radiotherapy was delivered using megavoltage equipment. Electrons were allowed to treat the posterior neck if necessary to reduce spinal cord dose. The prescribed tumor dose was 62–70 Gy in 31–35 fractions. Median overall treatment time was 46 and 39 days in the 5 and 6 fractions/week arms, respectively. Patients in DAHANCA 7 (all sites but glottic larynx) received nimorazole administered orally at a dose of 1200 mg/m2 body surface together with the first 30 radiation treatments. Patients did not receive chemotherapy nor EGFR-inhibitors.
Patients with severe dysphagia received nutritional support, most commonly by percutaneous endoscopic gastrostomy (PEG) tubes or nasogastric tubes. No efforts were made to spare swallowing related structures.
Endpoints and statistical analysis
All diagnostic, therapeutic and follow-up data were registered locally in the contributing treatment departments and collected and processed by the DAHANCA data center.
Data on early dysphagia (from start of radiotherapy to three months after the end of radiotherapy) and late dysphagia (from three months after end of radiotherapy to the end of follow-up) were scored on an arbitrary scale from zero to four. For the current analysis, dichotomized maximum scores were used. Severe dysphagia was defined as dysphagia grade 3 or 4 (liquid food only or worse), i.e. equal to the standard indication for tube feeding. Baseline dysphagia was divided into grade 0–1 versus grade 2–4. The reason for this division was to explore whether dysphagia present before treatment had any impact on the risk of developing dysphagia following treatment. No interpolations or other substitutes for missing data were used except for baseline dysphagia which was defined as dysphagia scored before treatment (week 0) or week 1 if information from week 0 was missing. If the patient was not scored in week 0 or 1, baseline dysphagia was defined as missing. Prevalence of a given reaction at a specific time was defined as the proportion of patients scored as having a specific grade of reaction relative to the total number of patients assessed at that time.
Univariate and multivariate logistic regression analyses were performed to describe the association between baseline and treatment characteristics against the risk of acute and late dysphagia. Risks were expressed as odds ratios with 95% confidence intervals (95% CI). First, a univariate analysis was performed; data were analyzed and compared using the χ2-test. All factors were further analysed in a multivariate logistic regression analysis with and without backward selection methods. A p-value ≤ 0.05 was considered significant; all tests were two-sided.
We calculated individual risk scores identical to the scores published by Langendijk et al. [Citation4] by summing up the following risk points for each individual patient: T-classification (T3 = 4 points, T4 = 4 points), neck irradiation (bilateral neck irradiation = 9 points), weight loss (1–5% = 5 points, 5–10% = 5 points, > 10% = 7 points), primary tumor site (oropharynx = 7 points, nasopharynx = 9 points) and treatment modality (accelerated RT = 6 points, concomitant chemotherapy = 5 points). In the present study, weight loss was not described at baseline but patients with baseline dysphagia above 1 was allocated to 5 points, since modification of diet might have influenced the patients weight in most cases. No patients received concomitant chemotherapy. The patients were divided into three risk groups for both acute and late dysphagia (low, intermediate and high risk) based on their risk score. Low risk reflected a risk score below 10, intermediate risk reflected a risk score between 10 and 18 and high risk reflected a risk score above 18.
Data were analyzed using SPSS for Windows, version 18.
Results
Prevalence of dysphagia
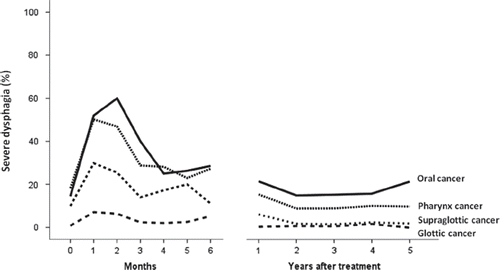
Acute dysphagia according to DAHANCA grades 1, 2, 3 and 4 occurred in 83%, 71%, 43% and 23%, respectively. Severe dysphagia, defined as liquid food only or worse (grade 3 or 4) occurred in 47% and 38% of patients receiving accelerated or conventional radiotherapy, respectively (p = 0.001). At one, two, three, four and five years the prevalence of chronic dysphagia greater than grade 0, was 46%, 32%, 29%, 24%, 23%, respectively with no difference between 5 and 6 fractions. The prevalence of severe dysphagia over time in different sites is presented in ; the prevalence was highest during and immediately after treatment and stable in the remaining follow-up period. There was a strong correlation between the peak incidence (grade) of acute dysphagia and development of late dysphagia. A total of 350 patients (24%) experienced severe xerostomia; a significant association was found between severe xerostomia and both severe acute and late dysphagia (p < 0.0001). Treatment with nimorazole did not influence the prevalence of severe acute or late dysphagia.
Univariate analysis
In univariate analysis () the following factors were significantly associated with severe acute dysphagia: gender (female vs. male), age (over median vs. below), site (non-glottic cancer vs. glottis cancer), T- stage (T3–4 vs. T1–2), N-classification (node positive vs. node negative), clinical stage (III–IV vs. I–II), fractions per week (6 vs. 5) and baseline dysphagia (above 1 vs. below). The following factors were significantly associated with severe late dysphagia: gender (female vs. male), age (over median vs. below), site (non-glottic cancer vs. glottis cancer), T-stage (T3–4 vs. T1–2), N-classification (node positive vs. node negative), clinical stage (III–IV vs. I–II) and baseline dysphagia (above 1 vs. below).
Table II. Univariate analysis of risk factors.
Multivariate analysis
In multivariate analysis ( and ) a number of independent risk factors were identified for severe acute dysphagia: non-glottic cancer (OR = 6.46), T3–T4 tumors (OR = 1.54), N-positive disease (OR = 1.99), baseline dysphagia > 1 (OR = 2.10), age > median (62 years) (OR = 1.45) and accelerated radiotherapy (OR = 1.82). For severe late dysphagia fewer risk factors were identified. Only non-glottic cancer (OR = 6.97), T3–T4 tumor (OR = 2.01), N- positive disease (OR = 1.62) and baseline dysphagia > 1 (OR = 2.29) were found to be prognostic factors.
Table III. Multivariate analysis of risk factors for severe acute and late dysphagia. Odds ratios (OR), 95% confidence intervals (95% CI) and p-values.
Table IV. Multivariate analysis of risk factors for severe acute and late dysphagia using backward selection. Odds ratios (OR), 95% confidence intervals (95% CI) and p-values.
If patients with baseline dysphagia above 1 were excluded (n = 120), the significant prognostic factors in univariate analysis for both severe acute and severe late dysphagia were the same except for age. Similarly, in multivariate analysis of severe acute dysphagia non-glottic cancer, high T- and N-classification and accelerated radiotherapy remained independent risk factors. For severe late dysphagia only site and high T-classification was found to have independent prognostic importance.
Validation of predictive model by Langendijk et al.
The total population was divided into groups with low, intermediate and high risk as described above for both acute dysphagia and late dysphagia. The proportion of patients developing severe acute dysphagia was 24%, 60% and 73% (p < 0.0001) in the low-, intermediate- and high-risk group, respectively and the proportion of patients developing severe late dysphagia was 6%, 29% and 38% (p < 0.0001) in the low-, intermediate- and high-risk group for late dysphagia, respectively.
Discussion
The current study has established a multivariate prognostic model for acute and late dysphagia after RT using data from the DAHANCA 6&7 trial. Factors associated with severe acute dysphagia were non-glottic cancer, advanced stage, baseline dysphagia, high age and accelerated radiotherapy. For severe late dysphagia the prognostic factors were non-glottic cancer, advanced stage and baseline dysphagia.
The main strength of the study is the volume of information prospectively collected from more than 1400 patients during substantial follow-up as part of a randomized trial. It is a limitation that the original study was conducted before IMRT and chemotherapy became standard. Also, factors like smoking status, co-morbidity and enteral feeding which are believed to be important factors for dysphagia, have not been collected consistently.
International scoring systems used for radiation-induced dysphagia differ; in addition to the DA-HANCA morbidity scoring system used in this study, the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer system (RTOG/EORTC), the Common Toxicity Criteria for Adverse Events, or the Worlds Health Organization system (CTCAE/WHO) and the Subjective, Objective, Management, Analytic/Late Effects Normal Tissue (SOMA/LENT) have been used for physician-based scoring of early and late dysphagia. The different scoring systems cannot be directly interchanged [Citation5], leading to difficulties when comparing studies. The DAHANCA morbidity scoring system for dysphagia used in this study distinguishes between both the degree of dysphagia and the impact on the patient's life. It does not include aspiration as a swallowing disability which is a potential limitation since patients are at risk of developing aspiration pneumonia [Citation6]. The DAHANCA dysphagia score correlates well with the EORTC H&N35-subscale “Swallowing” but like other observer-based scoring systems the clinicians tend to underestimate the patients complaints [Citation7].
The available studies on dysphagia are difficult to compare since there are considerable differences in endpoints, examined variables, time to assessment and factors influencing dysphagia like chemotherapy, fractionation schedule and radiation techniques. Most studies assess either acute [Citation2,Citation8–12] and/or late dysphagia [Citation4,Citation13–17]; the latter mostly recorded at six months post-RT. Our data on prevalence of dysphagia showed a decline up to two years after treatment and it is thus possible that late morbidity recorded at six months post-RT may not be representative.
To our knowledge there are no other studies in this field with the same number of patients and the same length of follow-up. The largest study on late dysphagia so far was published by Langendijk et al. [Citation4], based on data from 529 patients. Patients were treated with curative radiotherapy either alone or in combination with surgery or chemotherapy. Patients with late dysphagia grade 2 or higher at baseline were excluded. They found a prevalence of grade 2–4 RTOG swallowing dysfunction at six months at 23% and swallowing dysfunction at six months was prognostic for swallowing dysfunction in the subsequent time period. In multivariate analysis T-classification (T3–T4) and site (oropharynx or nasopharynx) together with bilateral irradiation, weight loss, accelerated radiotherapy and concomitant chemotherapy were identified as individual risk factors for swallowing dysfunction six months after completion of radiotherapy. T-classification and site as risk factors are in agreement with our data. The observed values from the present study fitted well with the published normal tissue complication probability (NTCP) values of < 10% for the low-risk group, 10–30% for the intermediate-risk group and > 30% for the high-risk group for late dysphagia indicating that the model works well for late dysphagia. Similarly, the risk scores also separated patients with regard to differences in the relative risk of acute dysphagia, in agreement with the observations of Langendijk et al. [Citation18].
Other studies of late dysphagia have also found that T-classification [Citation14,Citation16], site (different subsites) [Citation14–16,Citation19], age [Citation14,Citation15] and baseline swallowing function [Citation15] if examined are important risk factors. Treatment modality (± chemotherapy among others) correlates with severe dysphagia in some studies [Citation15,Citation16] but many studies were not able to identify concomitant chemotherapy as a risk factor because of homogeneity of the patients in each study; either none or all patients received chemotherapy. In the current study no patients received chemotherapy.
Risk factors for severe acute swallowing dysfunction in our study included site (different subsites), age, T- and N-classification, in concordance with other studies [Citation8–10,Citation19]. Baseline dysphagia and fractions per week were also significant in the present study, but have not been studied in details in other studies [Citation2,Citation10,Citation12]. Logemann et al. [Citation2] found that treatment increased the frequency, severity and clinical manifestations of the swallowing disorders seen before treatment.
It has been shown that radiation dose to the pharyngeal constrictors and other anatomical areas are important predictors for dysphagia and studies aiming at identifying dose-volume characteristics and specific organs at risk for swallowing have been published [Citation20–24]. A reduction in the radiation dose to the swallowing structures with IMRT [Citation25,Citation26] or proton radiotherapy [Citation27–29] without compromising tumor dose seems to be possible without exceeding other normal tissue constraints. Dosimetric factors were not considered in this analysis; all patients were treated with standard two-dimensional (2D) technique resulting in homogenous dose to the swallowing structures and it was not possible to extract individual dose-volume parameters. The results from the present study add to the knowledge of factors important for dysphagia after radiotherapy and may be important especially for centers not using neither IMRT nor chemotherapy and may be used in the identification of risk groups. Identification of risk groups is important for studies of prophylactic measures, e.g. dysphagia-sparing radiotherapy [Citation28] or prophylactic placement of feeding tubes as well as in selecting patients for rehabilitation, e.g. swallowing exercises [Citation30] in high-risk patients.
Conclusion
Prognostic models were established to characterize patients at risk of developing acute or late dysphagia after RT in the DAHANCA 6&7 trial. The results may be useful to identify patients who are at high risk of dysphagia and thus candidates for prophylactic measures against swallowing dysfunction.
Conflicts of interest: L Specht: Consultancy and member of advisory board/PI: Merck serono and Takeda Millennium. Dose steering committee member: Fresenius Biotech. PI: Boehringer Ingelheim. Payment for lectures and travel/accomodation for international conferences: Roche. Other Conflicts of interest: None.
References
- Mittal BB, Pauloski BR, Haraf DJ, Pelzer HJ, Argiris A, Vokes EE, et al. Swallowing dysfunction – preventative and rehabilitation strategies in patients with head-and-neck cancers treated with surgery, radiotherapy, and chemotherapy: A critical review. Int J Radiat Oncol Biol Phys 2003; 57:1219–30.
- Logemann JA, Rademaker AW, Pauloski BR, Lazarus CL, Mittal BB, Brockstein B, et al. Site of disease and treatment protocol as correlates of swallowing function in patients with head and neck cancer treated with chemoradiation. Head Neck 2006;28:64–73.
- Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 2003;362:933–40.
- Langendijk JA, Doornaert P, Rietveld DH, Verdonck- de Leeuw IM, Leemans CR, Slotman BJ. A predictive model for swallowing dysfunction after curative radiotherapy in head and neck cancer. Radiother Oncol 2009;90:189–95.
- Golen M, Skladowski K, Wygoda A, Przeorek W, Pilecki B, Maciejewski B, et al. A comparison of two scoring systems for late radiation toxicity in patients after radiotherapy for head and neck cancer. Reports Pract Oncol Radiother 2005;10:179–92.
- Mortensen HR, Jensen K, Grau C. Aspiration pneumonia in patients treated with radiotherapy for head and neck cancer. Acta Oncol 2013;52:270–6.
- Jensen K, Bonde JA, Grau C. The relationship between observer-based toxicity scoring and patient assessed symptom severity after treatment for head and neck cancer. A correlative cross sectional study of the DAHANCA toxicity scoring system and the EORTC quality of life questionnaires. Radiother Oncol 2006;78:298–305.
- Koiwai K, Shikama N, Sasaki S, Shinoda A, Kadoya M. Risk factors for severe dysphagia after concurrent chemoradiotherapy for head and neck cancers. Jpn J Clin Oncol 2009;39:413–7.
- Mangar S, Slevin N, Mais K, Sykes A. Evaluating predictive factors for determining enteral nutrition in patients receiving radical radiotherapy for head and neck cancer: A retrospective review. Radiother Oncol 2006;78:152–8.
- Poulsen MG, Riddle B, Keller J, Porceddu SV, Tripcony L. Predictors of acute grade 4 swallowing toxicity in patients with stages III and IV squamous carcinoma of the head and neck treated with radiotherapy alone. Radiother Oncol 2008;87:253–9.
- Salama JK, Stenson KM, List MA, Mell LK, MacCracken E, Cohen EE, et al. Characteristics associated with swallowing changes after concurrent chemotherapy and radiotherapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 2008;134:1060–5.
- Nourissat A, Bairati I, Samson E, Fortin A, Gelinas M, Nabid A, et al. Predictors of weight loss during radiotherapy in patients with stage I or II head and neck cancer. Cancer 2010;116:2275–83.
- Ghadjar P, Simcock M, Zimmermann F, Betz M, Bodis S, Bernier J, et al. Predictors of severe late radiotherapy-related toxicity after hyperfractionated radiotherapy with or without concomitant cisplatin in locally advanced head and neck cancer. Secondary retrospective analysis of a randomized phase III trial (SAKK 10/94). Radiother Oncol 2012;104: 213–8.
- Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol 2008;26:3582–9.
- Caudell JJ, Schaner PE, Meredith RF, Locher JL, Nabell LM, Carroll WR, et al. Factors associated with long-term dysphagia after definitive radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2009;73:410–5.
- Chen AM, Li BQ, Jennelle RL, Lau DH, Yang CC, Courquin J, et al. Late esophageal toxicity after radiation therapy for head and neck cancer. Head Neck 2010;32: 178–83.
- Rutten H, Pop LA, Janssens GO, Takes RP, Knuijt S, Rooijakkers AF, et al. Long-term outcome and morbidity after treatment with accelerated radiotherapy and weekly cisplatin for locally advanced head-and-neck cancer: Results of a multidisciplinary late morbidity clinic. Int J Radiat Oncol Biol Phys 2011;81:923–9.
- Koiwai K, Shikama N, Sasaki S, Shinoda A, Kadoya M. Validation of the Total Dysphagia Risk Score (TDRS) as a predictive measure for acute swallowing dysfunction induced by chemoradiotherapy for head and neck cancers. Radiother Oncol 2010;97:132–5.
- Ottosson S, Zackrisson B, Kjellen E, Nilsson P, Laurell G. Weight loss in patients with head and neck cancer during and after conventional and accelerated radiotherapy. Acta Oncol 2013;52:711–8.
- Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: Which anatomic structures are affected and can they be spared by IMRT?Int J Radiat Oncol Biol Phys 2004;60:1425–39.
- Bhide SA, Gulliford S, Kazi R, El-Hariry I, Newbold K, Harrington KJ, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol 2009;93:539–44.
- Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2010;76:403–9.
- Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: Frequency, intensity and correlation with dose and volume parameters. Radiother Oncol 2007;85:74–82.
- Sanguineti G, Rao N, Gunn B, Ricchetti F, Fiorino C. Predictors of PEG dependence after IMRT+/-chemotherapy for oropharyngeal cancer. Radiother Oncol 2013:107:300–4.
- Eisbruch A, Levendag PC, Feng FY, Teguh D, Lyden T, Schmitz PI, et al. Can IMRT or brachytherapy reduce dysphagia associated with chemoradiotherapy of head and neck cancer? The Michigan and Rotterdam experiences. Int J Radiat Oncol Biol Phys 2007;69:S40–2.
- van der Laan HP, Christianen ME, Bijl HP, Schilstra C, Langendijk JA. The potential benefit of swallowing sparing intensity modulated radiotherapy to reduce swallowing dysfunction: An in silico planning comparative study. Radiother Oncol 2012;103:76–81.
- van der Laan HP, van de Water TA, van Herpt HE, Christianen ME, Bijl HP, Korevaar EW, et al. The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: A planning comparative study. Acta Oncol 2013;52:561–9.
- Langendijk JA, Lambin P, De RD, Widder J, Bos M, Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model-based approach. Radiother Oncol Epub 2013:107:267–73.
- van der Laan HP, Gawryszuk A, Christianen ME, Steenbakkers RJ, Korevaar EW, Chouvalova O, et al. Swallowing-sparing intensity-modulated radiotherapy for head and neck cancer patients: Treatment planning optimization and clinical introduction. Radiother Oncol Epub 2013:107:282–7.
- Mortensen HR, Jensen K, Aksglæde K, Lambertsen K, Eriksen E, Grau C. Prophylactic systematic swallowing exercises in radical radiotherapy in head and neck cancer patients – a randomized trial. Submittet(in press).