Abstract
Purpose. To study if partnership modifies the effect of gastrointestinal symptoms on quality of life after radiation therapy for prostate cancer. Material and methods. Using a study-specific questionnaire we conducted a cross-sectional follow-up of the occurrence gastrointestinal symptoms and quality of life after radiation therapy for prostate cancer. We obtained information from 874 prostate cancer survivors treated with radiation therapy at the Sahlgrenska University Hospital, Sweden between 1994 and 2006. In this paper we describe how partnership status affects the association between gastrointestinal symptoms and quality of life. Results. We found that unpartnered men with gastrointestinal symptoms reported a lower quality of life than unpartnered men without such symptoms. Unpartnered men with symptoms had an excess risk of low quality of life compared with unpartnered men without symptoms for those experiencing altered composition of stools, prevalence ratio 3.8 (95% CI 1.1–13.1), leakage, 3.6 (1.3–10.1), sensory bowel symptoms, 4.5 (1.6–12.8), and for urgency, 4.2 (1.2–15.1). We also found that unpartnered men with symptoms had an excess risk of low quality of life compared with partnered men with symptoms for those experiencing altered composition of stools, prevalence ratio 2.9 (95% CI 1.4–5.8), leakage 2.8 (1.2–6.4), sensory bowel symptoms 3.4 (1.5–7.4), urgency 2.6 (1.2–5.8), and for any gastrointestinal symptom 2.5 (1.3–4.9). Conclusion. Unpartnered men may represent a group that is specifically vulnerable to the distressful effects of gastrointestinal symptoms after radiation therapy for prostate cancer.
Pelvic radiation therapy increases overall survival in men with advanced localized prostate cancer [Citation1]. However, for some prostate cancer survivors cure comes at a cost: radiation-induced gastrointestinal symptoms [Citation2]. Fifty percent of prostate cancer survivors treated with radiation therapy report that such gastrointestinal symptoms affect quality of life and for 20–40% this effect is moderate to severe [Citation3].
Lack of support can reduce quality of life for men diagnosed with prostate cancer [Citation4,Citation5]. Unpartnered men are less content with their life and report a poorer psychological and overall well-being compared with partnered men when facing a prostate cancer diagnosis [Citation4–7]. However, it is not known if unpartnered men are more vulnerable to the late side effects after prostate cancer treatment.
In a cross-sectional follow-up in 2008, we sent a study-specific questionnaire to all men who received radiation therapy for prostate cancer at the Sahlgrenska University Hospital, Gothenburg, Sweden, between 1994 and 2006. The questionnaire assessed gastrointestinal symptoms and quality of life after radiation therapy for prostate cancer. In this paper we report the results on partnership status as an effect-modifier for the association between gastrointestinal symptoms (exposure) and quality of life (outcome).
Material and methods
Study population
In 2008, using information from the Swedish Total Population Register and computerized hospital medical records, we identified 985 living prostate cancer survivors consecutively treated with radiation therapy between 1994 and 2006 at the Sahlgrenska University Hospital, Gothenburg, Sweden. Eligible men were 80 years old or younger, had no diagnosed distant metastases, and were resident in Sweden at the time of follow-up. The Regional Ethical Review Board in Gothenburg approved the project.
Radiation therapy
External beam radiation therapy was based on computerized tomography with the patient in supine position. The planning target volume consisted of the prostate or the post-operative prostatic region with 20 mm margin except for the rectal margin, which was 15 mm or, at most, half the cross-sectional rectal area. The patient was treated using a conformal three-field technique with one anterior and two lateral wedged fields with 11 or 15 MV photon energy. Brachytherapy was planned and delivered under the guidance of transrectal ultrasound with the patient in lithotomy position. The dose distribution was optimized to cover the prostate plus 2 mm margin with the prescribed dose while keeping the absorbed dose to the anterior rectal wall below a 6 Gy per fraction.
The questionnaire
The study-specific questionnaire was in Swedish and has been described in detail elsewhere [Citation2]. It was designed to survey patient-reported symptoms after radiation therapy for prostate cancer, several of which have not been reported previously. It was developed according to the well-founded method established at the Division of Clinical Cancer Epidemiology at the University of Gothenburg in Göteborg and the Karolinska Institutet in Stockholm, Sweden, documented in more than 100 published articles [Citation7–11]. Briefly, we started out by making structural assessments of four previously validated questionnaires from other projects at our division concerning pelvic symptoms after radiation therapy and/or surgery for prostate cancer [Citation9–12]. From these, we conceptualized clear-cut atomized definitions of relevant symptoms, which we used to construct single- item questions in the questionnaire. In addition, we conducted four semi-structured interviews with irradiated prostate cancer survivors to certify that we did not miss any common symptoms. Face-validity was assured by having 15 men (10 prostate cancer survivors) complete the questionnaire separately with one investigator (DA) present. During and after this procedure, the investigator discussed the questionnaire with each man noting any issues regarding confusion, misinterpretation, negative apprehension or lack of understandability of specific questions. After each completed questionnaire the investigator edited all questions that were misinterpreted or not directly understood. We repeated this procedure until no further changes were suggested. We also consulted external experts in clinical radiation oncology and urology to review the questionnaire. Finally, we conducted an unpublished preparatory study including 30 prostate cancer survivors to test for logistics and participation rate. Omerov et al. give a thorough description of a similar process from our division for a questionnaire concerning suicide-bereaved parents in Sweden [Citation13].
Data collection
We sent out the questionnaire between February and November 2008. It included 165 questions on pelvic symptoms, demographic data, co-morbid diseases, other treatments, quality of life, and physical health. In the questionnaire responders assessed 34 questions that specifically reflected the occurrence of gastrointestinal symptoms the previous six months. Responders also assessed one question reflecting quality of life the previous six months on a visual digital scale ranging from 0 to 6, where 0 meant “worst possible” and 6 meant “best possible”. Responders reported their current civil status by stating if they were “Married or cohabiting”, “Living alone, without a partner”, or “Living alone but having a partner (live-apart)”.
Statistical analyses
All calculations were done in SAS 9.2 for Windows (SAS Institute Inc., Cary, NC, USA) and Stata/IC 11.2 for Mac (StataCorp., College Station, TX, USA). We evaluated differences in the characteristics of the study population at follow-up using a non-parametric Kruskal-Wallis test for age and time to follow-up and a χ2-test for the remaining variables. We dichotomized outcome variables to get a balance between clinical relevance and background noise (sporadic symptom occurrence) and to reduce the risk of recall bias by reporting more frequently occurring symptoms [Citation8–12]. We considered 0–2 on the visual digital scale as indicating a low quality of life.
We assigned each of the 34 symptom questions to one out of six categories, based on common features of clinical presentation (). For each symptom category we divided the responders into the following four groups based on symptom occurrence and stratified for partnership status as an effect modifier: A) symptom/unpartnered, B) no symptom/unpartnered, C) symptom/partnered, and D) no symptom partnered. To estimate differences between these four groups (A–D) we used a log-binomial model (GENMOD procedure in SAS 9.2 for Windows) to calculate the prevalence ratio of low quality of life. We also calculated the prevalence ratios of gastrointestinal symptoms between partnered and unpartnered men to assure that the combined symptom/partner groups (A–D) were not subjected to bias due to differences in symptom occurrence. We also included the possible confounders’ age, comorbid diseases (cardiovascular disease, diabetes, heart failure, neurological disease, psychiatric disease, pulmonary disease, and rheumatic disease), educational level, radiation modality, smoking, and time to follow-up in a multivariable log-binomial model to produce adjusted prevalence ratios. We considered a confidence interval not including 1.0 or a p-value of 0.05 or less as indicating statistical significance.
Table I. Categories of gastrointestinal symptoms*.
Results
Altogether 874 (89%) of the 985 eligible prostate cancer survivors returned a filled in questionnaire. We found a significant difference between the four groups (A–D) for age (p = 0.009), comorbid diseases (p = 0.05), radiation modality (p = 0.02), smoking (p = 0.022) and widowers (< 0.001) (). We found no difference in the prevalence of gastrointestinal symptoms among unpartnered men compared with partnered men ().
Table II. Characteristics of the study-population.
Table III. Prevalence of gastrointestinal symptoms among prostate cancer survivors with or without partner, who had been treated at the Sahlgrenska University Hospital, Gothenburg, Sweden between 1994 and 2006.
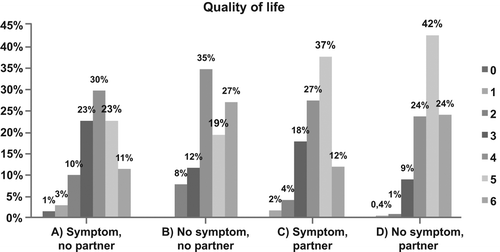
The men in group A (symptom/unpartnered) reported a lower quality of life compared with the men in group B (no symptom/unpartnered) for the symptom categories: altered composition of stools, prevalence ratio 3.8 (95% CI 1.1–13.1), leakage 3.6 (1.3–10.1), sensory bowel symptoms 4.5 (1.6–12.8), and for urgency 4.2 (1.2–15.1) (). However, for any gastrointestinal symptom the difference was not statistically significant.
Table IV. Prevalence ratio of low* quality of life, among irradiated prostate cancer survivors with or without a partner, who had been treated at the Sahlgrenska University Hospital, Gothenburg, Sweden between 1994 and 2006.
The men in group A (symptom/unpartnered) reported a lower quality of life compared with the men in group C (symptom/partnered) for the symptoms categories: altered composition of stools, prevalence ratio 2.9, (95% CI 1.4–5.8), leakage, 2.8, (1.2–6.4), sensory bowel symptoms, 3.4, (1.5–7.4), and urgency, 2.6, (1.2–5.8). For any gastrointestinal symptom, 14% of the men in group A reported a low quality of life compared with 6% of the men in group C prevalence ratio 2.5, (95% CI 1.3–4.9). For the groups as a whole, more men in group A (14%) reported a low quality of life compared with the men in group B (8%), group C (6%), and group D (1.4%) (). Adjusting for the possible confounders age, comorbid disease, educational level, radiation modality, smoking, and time to follow-up, in a multivariate analysis did not change the results substantially (Supplementary material Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.841988), nor did analyzing the exposure (gastrointestinal symptoms) without dichotomization and the outcome (quality of life) without trichotomization (data not shown).
Figure 1. Quality of life among 874 long-term prostate cancer survivors who had been treated with radiation therapy between 1994 and 2006. The results are presented according to partnership status (partnered/unpartnered) and presence of gastrointestinal symptoms (yes/no). Quality of life was assessed on a visual digital scale ranging from 0 to 6, where 0 denotes “worst possible” and 6 denotes “best possible”.
Discussion
Being unpartnered, especially if you are a man, can have a large negative impact on your quality of life, as can gastrointestinal symptoms after radiation therapy for prostate cancer. This study shows that unpartnered prostate cancer survivors experiencing these symptoms report a lower quality of life compared with unpartnered survivors without symptoms and partnered survivors with symptoms.
Our results add to current knowledge by suggesting that unpartnered prostate cancer survivors could represent a group of men specifically vulnerable to distress from gastrointestinal symptoms. Zhou and co-workers found an association between high levels of baseline social support and high ratings of “health-related quality of life” two years after surgery or radiotherapy for localized prostate cancer [Citation14]. The authors suggest that this association is partially mediated through lower perception of stress. They also found that a high level of baseline social support predicts a high level of well-being two years after treatment [Citation15]. A Danish study showed that prostate cancer survivors who lived alone had a higher risk of reduced quality of life compared with partnered men [Citation16]. Our data indicate that being afflicted with gastrointestinal symptoms could further aggravate this decline in quality of life.
We hypothesize that the emotional and physical support from a partner may enable partnered men to be more tolerant of gastrointestinal side effects compared to unpartnered men. This hypothesis is supported by data from other previously published studies on cancer patients and survivors. In a study on 1736 patients treated for head and neck cancers, Dilling et al. found that unpartnered males had inferior overall survival rates compared to partnered males, adjusted hazard ratio 1.20 (95% CI 1.09–1.28) [Citation17]. They hypothesize that partnered patients receive more support from their partners, both emotional and physical and therefore tolerate treatment better, leading to less treatment breaks and better outcomes. Wu et al. show that spouse timeline beliefs mediated the association between spouse treatment control beliefs and patient quality-of-life six months later [Citation18]. They argue that spouses’ beliefs likely shaped interactions between the couple, reinforced daily life activities and influenced coping behaviors that bolstered patients’ quality-of-life. Data from the Whitehall II study show that a low level of confiding and emotional support from the “closest person” predicts psychiatric morbidity after an “acute life event” [Citation19]. They report that for men, 92 % of the “closest persons” were a spouse, a partner or a cohabitee and that the perceived social support from this “closest person” had a larger effect on psychiatric morbidity than tangible or practical aspects of support. Furthermore, investigations of low-income, uninsured men with prostate cancer show that those who are partnered have better health and lower symptom distress than those who are unpartnered, pointing to the alleviating role of a partner for men in a vulnerable position [Citation20].
The strengths of this study include a large study population with a long time to follow-up and information on symptom background rates in a non-irradiated population. We used epidemiological methods adapted to the cancer survivorship field according to the hierarchical step-model for causation of bias [Citation21]. The Swedish Total Population Register enables us to follow-up all eligible survivors and together with a high participation rate and high response rates to individual questions this reduces the risk of selection-induced problems. The use of postal questionnaire minimizes the risk of interviewer-related problems. Although the treatment protocols remained stable over time, there may have been minor adjustments for which we lack data. However, we have no reason to believe that this would alter the overall result of this study.
Nevertheless, this study has limitations that could affect the results. We cannot exclude that there are unmeasured confounding factors associated with both the occurrence of gastrointestinal symptoms (exposure) and quality of life (outcome). It is possible that men with a lower quality of life overestimate the occurrence of gastrointestinal symptoms, thereby introducing a differential misclassification. Speaking against this, however, is the consistent difference we found in quality of life rating between men with or without symptoms among both unpartnered and partnered men. Moreover, we found no difference in reported symptom occurrence between unpartnered and partnered men.
Gastrointestinal symptoms can cause considerable psychosocial distress among men irradiated for prostate cancer [Citation3]. Our results indicate that, in a clinical situation with limited resources, we may consider prioritizing unpartnered men as having the largest benefit from interventions aimed at distressful consequences of gastrointestinal symptoms after prostate irradiation.
Supplementary material Table I
Download PDF (34.7 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): An open randomised phase III trial. Lancet 2009;373:301–8.
- Alsadius D, Hedelin M, Johansson K-A, Pettersson N, Wilderäng U, Lundstedt D, et al. Tobacco smoking and long-lasting symptoms from the bowel and the anal-sphincter region after radiotherapy for prostate cancer. Radiother Oncol 2011;101:495–501.
- Andreyev HJ, Wotherspoon A, Denham JW, Hauer-Jensen M. Defining pelvic-radiation disease for the survivorship era. Lancet Oncol 2010;11:310–2.
- Helgason ÁR, Dickman P, Adolfsson J, Steineck G. Emotional isolation: Prevalence and the effect on well-being among 50–80-year-old prostate cancer patients. Scand J Urol Nephrol 2001;35:97–101.
- Ko CM, Malcarne VL, Varni JW, Roesch SC, Banthia R, Greenbergs HL, et al. Problem-solving and distress in prostate cancer patients and their spousal caregivers. Support Care Cancer 2005;13:367–74.
- Mehnert A, Lehmann C, Graefen, Huland H, Koch U. Depression, anxiety, post-traumatic stress disorder and health-related quality of life and its association with social support in ambulatory prostate cancer patients. Eur J Cancer Care 2010;19:736–45.
- Eton D, Lepore SJ, Helgeson VS. Early quality of life in patients with localized prostate carcinoma: An examination of treatment-related, demographic, and psychosocial factors. Cancer 2001;92:1451–9.
- Steineck G, Bergmark K, Henningsohn L, Dickman PW, Al-Abany M, Helgason Á. Symptom documentation in cancer survivors as a basis for therapy modifications. Acta Oncol 2002;61:1130–7.
- Johansson E, Steineck G, Holmberg L, Johansson JE, Nyberg T, Ruutu M, et al. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: The Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol 2011;12:891–9.
- Dunberger G, Lind H, Steineck G, Waldenström AC, Nyberg T, al-Abany M, et al. Self-reported symptoms of fecal incontinence among long-term gynaecological cancer survivors and population-based controls. Eur J Cancer 2010;46:606–15.
- Nilsson AE, Schumacher MC, Johasson E, Carlsson S, Stranne J, Nyberg T, et al. Age at surgery, educational level and long-term urinary incontinence after radical prostatectomy. Br J Urol Int 2011;108:572–7.
- al-Abany M, Helgason AR, Cronqvist AK, Lind B, Mavroidis P, Wersäll P, et al. Toward a definition of a threshold for harmless doses to the anal-sphincter region and the rectum. Int J Radiat Oncol Biol Phys 2005;61:1035–44.
- Omerov P, Steineck G, Runeson B, Christensson A, Kreicbergs U, Pettersén R, et al. Preparatory studies to a population-based survey of suicide-bereaved parents in Sweden. Crisis 2013;34:200–10.
- Zhou ES, Penedo FJ, Lewis JE, Rasheed M, Traeger L, Lechner S, et al. Perceived stress mediates the effects of social support on health-related quality of life among men treated for localized prostate cancer. J Psychosom Res 2010;69: 587–90.
- Zhou ES, Penedo FJ, Bustillo NE, Rasheed M, Traeger L, Lechner S, et al. Longitudinal effects of social support and adaptive coping on the emotional well-being of survivors of localized prostate cancer. J Support Oncol 2010;8: 197–201.
- Dieperink KB, Hansen S, Wagner L, Johansen C, Andersen KK, Hansen O, et al. Living alone, obesity and smoking: Important factors for quality of life after radiotherapy and androgen deprivation therapy for prostate cancer. Acta Oncol 2012;51:722–9.
- Dilling TJ, Bae K, Paulus R, Watkins-Bruner D, Garden AS, Forastiere A, et al. Impact of gender, partner status, and race on locoregional failure and overall survival in head and neck cancer patients in three radiation therapy oncology group trials. Int J Radiat Oncol Biol Phys 2011;81:e101–9.
- Wu LM, Mohamed NE, Winkel G, Diefenbach MA. Patient and spouse illness beliefs and quality of life in prostate cancer patients. Psychol Health 2013;28:355–68.
- Stansfeld SA, Fuhrer R, Shipley MJ. Types of social support as predictors of psychiatric morbidity in a cohort of British Civil Servants (Witehall II Study). Psychol Med 1998;28: 881–92.
- Gore JL, Krupski T, Kwan L, Maliski S, Litwin MS. Partnership status influences quality of life in low-income, uninsured men with prostate cancer. Cancer 2005;104: 191–8.
- Steineck G, Hunt H, Adolfsson J. A hierarchical step-model for causation of bias-evaluating cancer treatment with epidemiological methods. Acta Oncol 2006;45:421–9.

