Abstract
Zoledronic acid (Zol) is frequently used for the treatment of bone disease in patients with multiple myeloma and breast cancer with metastasis to bone. Therefore, there is also an interest in finding the optimal dosing regimen to optimize effects, minimize side effects and reduce costs. In our phase II clinical trial we investigated the effect of Zol treatment on the serum levels of the bone markers collagen type 1 cross-linked C-telopeptide (CTX) and bone specific alkaline phosphatase (bALP) as well as on creatinine clearance (kidney function) in response to dosing and duration of treatment for each individual patient. Methods. We enrolled 30 multiple myeloma (MM) and 30 breast cancer (BC) patients whereof 10 of each had never received bisphosphonate and 20 had received at least six prior Zol treatments. Results. We found that Zol treatment strongly reduced CTX (Spearman's correlation, rs = −0.59, p = 0.0007) and bALP (Spearman's correlation, rs = −0.51, p = 0.0042) in MM patients while only CTX (Spearman's correlation, rs = −0.42, p = 0.024) was significantly affected in BC patients. Multiple linear regression analyses done on the entire cohort showed that the average time between each dose of Zol had the strongest impact on CTX (p < 0.001) and bALP (p = 0.011) levels while the total accumulated number of Zol infusions had a less pronounced effect on CTX levels (p = 0.015). In contrast, multiple linear regression analysis showed that the total number of Zol infusions had a strong negative impact on kidney function (p = 0.014) while the average time between each dose of Zol had no significant impact. Conclusion. Thus, if MM and BC patients are not treated regularly every month with Zol bone turnover is not fully suppressed, while prolonged treatment with zoledronic acid compromises kidney function. We believe that these data significantly contribute to the knowledge needed to find the optimal Zol treatment schedule.
Zoledronic acid (Zol) is a potent bisphosphonate which has been used for about a decade to treat bone disease in patients with multiple myeloma (MM) and breast cancer patients with metastasis to bone (BC). Recently, Zol has received additional attention since it was suggested that Zol has a direct influence on survival of MM patients [Citation1,Citation2] and possibly BC patients, the latter especially in postmenopausal women [Citation1,Citation3]. At the same time there is also a large interest in finding the optimal dosing regimen in order to optimize effects, minimize side effects and reduce costs [Citation4–8]. These considerations have become even more important since the mortality rate of these patient groups has improved over the recent years [Citation9–11] and it is therefore even more urgent to look at the quality of life of these patients. In this aspect, bone disease strongly influences quality of life and it is therefore very important to treat bone disease optimally to relief pain, prevent fractures but also optimally to leave the possibility that bone lesions may heal again once the cancer disease has been brought under control. Thus, it is on one hand important to keep the bone disease under control, but on the other hand also to avoid side effects and “freezing” of the bone since the latter can prevent healing and also potentially suppress the non-pathological bone turnover which is required for maintaining a strong bone and eventually preventing fractures [Citation12,Citation13].
In order to address these important issues the effect of different dosing regimens must be tested. In this context we set up a small trial which had the primary objective to compare the retention of a single Zol infusion in bisphosphonate naive or multiply treated MM and BC patients [Citation14]. Here we report on the secondary outcomes of this trial focusing on the effect of Zol on bone markers and kidney function.
Patients and methods
Approval of clinical trial
This study was conducted as a prospective non- randomized phase II clinical trial in accordance with the Helsinki declaration and “Good Clinical Practice” guidelines and was monitored by the GCP-unit at Odense University Hospital, Denmark. It was approved by the Danish Medicines Agency, the Regional Ethical Committee, and the Danish Data Protection Agency. All patients gave informed consent. The study received EudraCT number 2007-003777-13 and trial registration number NCT00760370 at ClinicalTrials.gov.
Patients
A total of 60 patients were included in the trial – 30 MM and 30 BC patients. Ten patients from each disease group had never received any BP previously and 20 patients had received at least six previous treatments with Zol for treatment of bone disease due to the current malignancy (Please refer to ). The time intervals between treatments for each individual patient prior to inclusion in the protocol were decided upon by the responsible physicians and noted in the CRF. The groups which had received Zol at least six times had received a median of 9 (MM) or 9.5 (BC) prior Zol treatments and the time between treatments was on average/median 1.4/1.0 (MM) and 1.5/1.2 (BC) months and with a range within the cohort of 1–3.3 (MM) and 0.9–3.2 (BC) months. Zol was given as an i.v. dose of 4 mg infused over 15 min. In the protocol the effect of a single Zol infusion of 4 mg was followed.
Table I. Patient Characteristics.
Inclusion criteria were: patients diagnosed with MM or BC with metastasis to bone, scheduled for Zol treatment, women in menopause (10–12 months after last menstrual cycle), men 50 years or older. Both patient categories should show clear signs of bone lesions by x-ray or CT scan. Important exclusion criteria were: patients under the age of 18, women receiving hormone therapy which induces menopause, women receiving estrogen substitution therapy or patients that had received treatment with any BP prior to the current malignant condition. For patient characteristics please refer to . Patients with MM were grouped as: 1) active myeloma encompassing newly diagnosed patients plus patients with PD; or 2) myeloma in remission (PR plus CR) () according to the IMWG criteria of response [Citation15]. Patients with BC were evaluated according to RECIST 1.0 criteria [Citation16]; all patients had PD (). Creatinine clearance was calculated according to Cockcroft and Gault [Citation17].
We have previously reported on the primary end-point of this trial (retention of Zol) [Citation14] and here we report on the secondary end-points comparing the effects of Zol on bone serum markers collagen type 1 cross-linked C-telopeptide (CTX), for bone resorption, and bone specific alkaline phosphatase (bALP), for bone formation, and how the difference in dosing between these patients, influenced their bone marker levels but also kidney function.
Bone markers
Blood samples were taken from fasting patients on day 0 just prior to their protocol related Zol-infusion and 14 days after the infusion. bALP levels were determined on blood samples collected in a non-coated tube while the samples for CTX measurement were coated with EDTA. The samples were frozen at −80°C until further processing. Concentrations were measured by full automatic ELISA. bALP was measured using Alisei (Seac Radim Group) and CTX on a Modular E. (Roche). ELISA analyses were done according to the instructions by the supplier: bALP ELA kit (Quidel Corp.); CTX-1 kit (Roche).
Whole body retention of Zol
Analysis of Zol concentration in urine samples was performed by the external contractor, SGS Cephac (Saint Benoit, France) through the use of a specific radioimmunoassay based on the competitive binding of 125I-labeled Zol and unlabeled Zol (as a standard) or urine samples to a polyclonal rabbit anti- zoledronic acid antibody coated microtiter plate as described previously [Citation14].
Statistics
Statistical tests for normal distribution, t-tests, linear regression and correlation analysis [Spearman's rank correlation test (rs)] were done using the GraphPad Prism 4.01 software (GraphPad Software Inc., La Jolla, USA). For these tests a p-value ≤ 0.05 was considered as significant.
For multiple linear regression analyses and likelihood ratio tests StataIC 12.1 (Stata Corp., College Station, USA) was used. To ensure that no multi co-linearity existed between predictor variables, variance inflation factors (VIFs) were computed for each predictor variable within the data set. Because no VIF was larger than 3, we did not exclude any predictor variable. When necessary (please refer to figure legends) a Box-Cox power transformation was performed to obtain normality. Variance homogeneity of the standardized residuals was confirmed. To find the best predicting variables for either CTX, bALP or creatinine clearance, the least significant variables from the dataset were removed step by step. Likelihood ratio tests were used to ensure that the predictive value of the dataset was not lost in this process. This was done until the further removal of variables in a likelihood ratio test resulted in a p-value ≤ 0.05. In this case the new model was rejected and the previous model was accepted as the best model to predict the dependent variable.
Results
Zol treatment more potently reduces CTX-levels in MM than BC patients
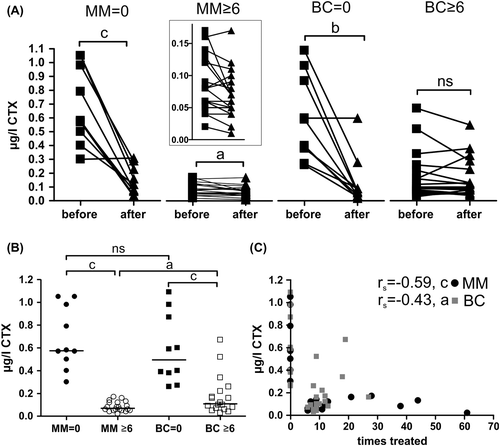
As expected Zol had a fast acting (< 14 days) effect on CTX levels and reduced the initial levels considerably within 14 days. However, note that one patient from both the MM and BC Zol-naive groups apparently did not respond to treatment (). In the two groups that had received at least six previous treatments it is seen that although the CTX-levels were already strongly reduced in these MM patients (MM ≥ 6) an additional infusion significantly reduced the levels further, while it was not the case in BC patients ( ≥ 6). Furthermore, CTX was more strongly suppressed in MM ( ≥ 6) (median reduction of 0.505 μg/l, p < 0.0001) than in BC patients ( ≥ 6) (median reduction of 0.385 μg/l, p = 0.0003) which is also supported by the weaker correlation between CTX-levels and number of Zol treatments for BC (rs = −0.43, p = 0.041) compared to MM patients (rs = −0.59, p = 0.0007) ().
Figure 1. Bone resorption marker levels, CTX, in response to Zol treatment. CTX-levels: A) measured in blood samples taken immediately before and 14 days after protocol related infusion of 4 mg Zol according to the treatment groups. Lines represent responses of individual patients within 14 days (statistics: two-sided paired t-test). The insert represents an enlargement to better visualize the individual changes in the MM ≥ 6 group. The means for each before/after measurement were as follows: MM = 0 (n = 10), before = 0.68, after = 0.14; MM ≥ 6 (n = 20), before = 0.083, after = 0.067; BC = 0 (n = 10), before = 0.58, after = 0.13; BC ≥ 6 (n = 17), before = 0.18, after = 0.16. B) Average CTX levels in different patients groups (statistics: two-sided Mann Whitney test). MM = 0, n = 10; MM ≥ 6, n = 20; BC = 0, n = 10; BC ≥ 6, n = 18. C) CTX-levels of each individual patient according to the total number of previous Zol infusions (two-sided Spearman's correlation analysis (rs). MM, n = 30; BC, n = 28. Abbreviations used: a: p < 0.05, b: p < 0.01, c: p < 0.001, ns: not significant. Some patients were excluded from these analyses as indicated above because one or both blood samples by mistake were not measured according to the protocol guidelines.
Zol treatment more potently reduces bALP-levels in MM than BC patients
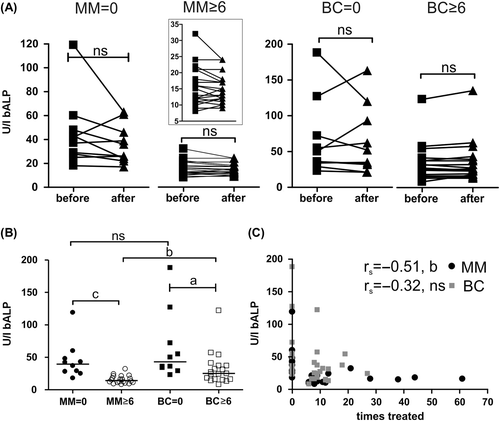
Within 14 days after Zol infusion bALP-levels were not found to be significantly reduced in any of the patient groups (). However, an analysis of treatment groups suggested that Zol treatment resulted in reduced bALP levels compared to the previously untreated patients (). But when bALP levels were related to the accumulated number of Zol treatments no significant correlation was found for BC (rs = −0.32, p = 0.091) while there was a significant correlation for MM patients (rs = −0.51, p = 0.0042) ().
Figure 2. Bone formation marker, bALP, in response to Zol treatment. bALP-levels: A) immediately before and 14 days after protocol related infusion of 4 mg Zol according to the treatment groups. Lines represent responses of individual patients (statistics: two-sided paired t-test). The insert represents an enlargement to better visualize the individual changes in the MM ≥ 6 group. The means for each before/after measurement were as follows: MM = 0 (n = 10), before = 44.9, after = 36; MM ≥ 6 (n = 20), before = 15.5, after = 14.7; BC = 0 (n = 10), before = 65, after = 63.2; BC ≥ 6 (n = 18), before = 33.2, after = 34.6. B) Average bALP-levels in different patients groups (statistics: two-sided Mann Whitney test). Vertical bars represent the median. MM = 0, n = 10; MM ≥ 6, n = 20; BC = 0, n = 10; BC ≥ 6, n = 19. C) bALP-levels of each individual patient according to the total number of previous Zol infusions (two-sided Spearman's correlation analysis). MM, n = 30; BC, n = 29. Abbreviations used: a: p < 0.05, b: p < 0.01, c: p < 0.001, ns: not significant. Some patients were excluded from these analyses as indicated above because one or both blood samples were not measured according to the protocol.
Zol dosing history affects bone marker levels
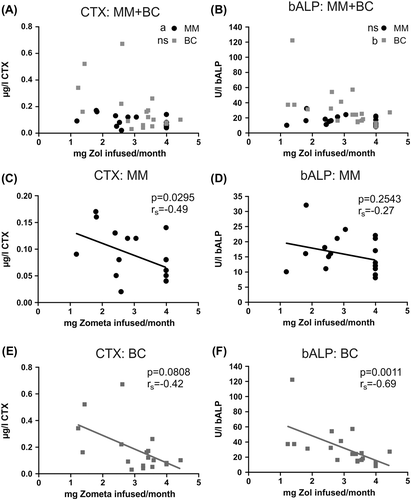
Comparing the prior Zol dosing history of the patients and their CTX () or bALP () levels we found that a small but significant increase in CTX-levels can be detected for MM patients the less frequently the patients were treated (rs = −0.49, p = 0.0295) ( and ) and with a near significant correlation for BC patients (rs = −0.42, p = 0.0808) ( and ). bALP levels also strongly correlated with prior dosing history and increased significantly for BC patients (rs = −0.69, p = 0.0011) when dosing was less frequent ( and ) while this was not found to be the case for MM patients (rs = −0.27, p = 0.27) ( and ).
Figure 3. Bone turn-over markers are elevated if patients are not treated regularly every month. A) CTX-levels in patients that have received at least 6 previous treatments and their prior dosing regimen (mg Zol infused/month before). 4 mg/month reflects on average a monthly regimen while 2 mg/month reflects dosing regimen every 2nd month and so forth. MM, n = 30; BC, n = 28. B) bALP-levels, as desribed in A. MM, n = 30; BC, n = 29. C-D) To improve visibility of the data, CTX (C) and bALP (D) data for MM patients are here shown on another scale, but are otherwise the same data as shown in A and B, respectively. E-F) CTX (E) and bALP (F) values for BC patients. Data are the same as shown in A and B, respectively. Statistics: two-sided Spearman correlation; a: p < 0.05, b: p < 0.01, c: p < 0.001, ns: not significant. Lines included represent trend-lines and do not indicate linear correlation. Some patients were excluded from these analyses as indicated above because one or both blood samples were not measured according to the protocol.
Zol dosing is a strong predictor of bone marker levels
In an attempt to find those variables that had the most impact on CTX-levels in our cohort we pooled seven independent variables into a multiple linear regression model in addition to the constant or intercept of the multiple linear regression called “_cons” (, left table). Using likelihood ratio tests we excluded those variables that had no significant impact on CTX-levels and found a new optimized linear model (, right table). This optimized model has an r2 of 0.706 and thereby explains a substantial fraction of the variation in CTX-levels observed in the cohort. It is worth noticing that the average Zol dose given (mg Zol infused/month, not all patients were treated regularly every month) is the most powerful predictor of CTX-levels and that it has a stronger influence than the accumulated number of treatments.
Figure 4. Zol dosing is the best predictor of bone marker levels. A) Dependent variable: CTX. An initial multiple linear regression model of power transformed CTX-levels was generated including seven different variables (in addition to the statistical variable “_cons”: constant or intercept) (table to the left) that could be considered to be of relevance for predicting CTX-levels in BC (n = 27) or MM (n = 30) patients. The table on the right hand side shows the final optimized model containing those variables and corresponding results of the multiple regression that best predict CTX-levels. In between the tables is shown the statistical parameters of the likelihood ratio test. B) Dependent variable: bALP. Same as in A, BC (n = 28) or MM (n = 30) patients. Some patients were excluded from these analyses as indicated above because not all variables included in the model had been determined correctly for all patients. WBrt, whole body retention.
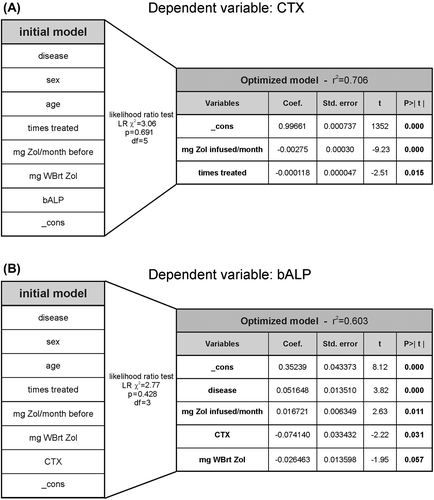
The corresponding optimized model for bALP contained four disease or treatment related variables (, right table). The most significant variable was found to be the disease of the patient (BC or MM). This most likely reflects that bALP levels were strongly reduced in MM patients after multiple Zol treatments while this was much less pronounced in BC patients (see ). As the second most important variable Zol dosing (mg Zol infused/month) was found to have a strong impact on bALP-levels. It should be noted that the accumulated number of treatments is not part of the optimized model highlighting that Zol dosing seems to be more important for predicting not only CTX but also bALP-levels. CTX-levels also have an impact, which most likely reflects some degree of coupling between resorption and formation despite of disease. Finally, the likelihood ratio test showed that also the whole body retention (WBrt) of Zol correlates with bALP levels although as an individual variable it is just outside of the significance definition.
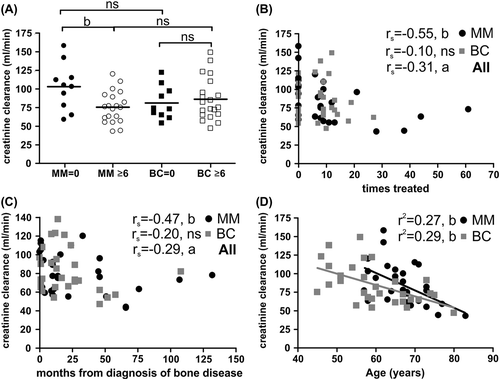
Prolonged Zol treatment decreases creatinine clearance
As it can be seen in the table of patient characteristics () and as it was commented in our prior publication [Citation14], creatinine levels were significantly reduced in the MM group that had received multiple Zol treatments while this was not the case in BC patients (, ). This suggested that multiple treatments with Zol may compromise kidney function as also emphasized by the negative correlation between the number of times each patient has been treated with Zol and the creatinine clearance (). However, we are also aware that other variables may explain the reduced creatinine clearance such as duration of bone disease (months from diagnosis of bone disease) () or age ().
Figure 5. Prolonged Zol treatment negatively affects creatinine clearance in MM patients. A) Average estimated creatinine clearance rates in different patient sub-groups (statistics: two-sided t-test). B) Estimated creatinine clearance rates as a function of the total number of previous Zol treatments (statistics: two-sided Spearman's correlation). C) Estimated creatinine clearance rates as a function of the time (months) from diagnosis of bone disease in MM or BC patients (statistics: two-sided Spearman's correlation). D) Estimated creatinine clearance rates as a function of the age of the patients (statistics: linear regression analysis). P-values: a: p ≤ 0.05, b: p < 0.01, ns: not significant.
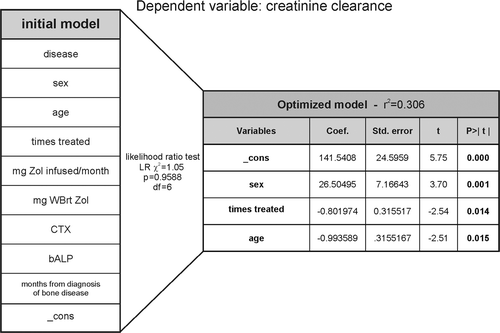
Thus, it is not straight forward to conclude if Zol treatment directly influences creatinine clearance. Therefore, we performed a multiple linear regression analysis including nine variables that may affect creatinine clearance along with the constant or intercept of the multiple linear regression called “_cons” (, left table). Using likelihood ratio tests we found sex, number of Zol treatments and age to be the most important determining variables for creatinine clearance in our cohort (, right table). This shows that although there are non-treatment related variables (such as age and sex) which influence creatinine clearance, the accumulated number of Zol treatments is indeed an independent predictor of creatinine clearance.
Figure 6. The accumulated number of Zol treatments is a strong predictor of creatinine clearance. Dependent variable: creatinine clearance. An initial multiple linear regression model was generated including 9 different variables (in addition to the statistical variable “_cons”: constant or intercept) (table to the left) that could be considered to be of relevance for predicting creatinine clearance in BC (n = 28) or MM (n = 30) patients. The table on the right hand side shows the final optimized model containing those variables that best predict creatinine clearance together with the statistical results of the multiple regression. In between the tables, is shown the statistical parameters of the likelihood ratio test. WBrt, whole body retention.
Discussion
There is an ongoing discussion about what is the optimal Zol dosing and duration of treatment for BC and MM patients [Citation4–8,Citation18,Citation19]. In our trial we have investigated and compared the effect of Zol on BC and MM patients after the first and after multiple treatments with Zol based on serum bone markers and serum creatinine levels. We believe that our data are particularly interesting in the context of the ongoing effort to optimize the use of Zol.
We found that Zol very efficiently reduced CTX levels in both MM and BC after just a single infusion, but also that repeated treatments, especially in MM patients, reduced CTX levels even further. Also, we found for both MM and BC patients, that those who were not treated with Zol every month had higher CTX and bALP-levels (only BC patients) than those receiving regular monthly treatment. This suggests that if patients are not treated every month bone turnover is not fully suppressed. Although the detected increases are minor we believe that they are of significance.
In a retrospective study of MM patients having received Zol treatment it was found that an increase of just 0.04 μg/l CTX from one month to the next was enough to predict ongoing osteolytic bone disease (as verified by x-ray) [Citation20]. This shows that if just a single local site escapes inhibition of Zol this results in small increases in CTX, but yet it is sufficient to cause a new osteolytic lesion that can be detected by x-ray. We therefore believe that the very strong suppression of CTX levels in our cohort is mainly due to inhibition of bone turnover in the skeleton in general and not necessarily at the specific pathological sites.
How could it be that Zol may inhibit bone turnover very efficiently in general but possibly less efficiently at the sites of pathological bone turnover? It may be that Zol is not bound to a sufficient extent at these sites in the first place as indicated by the following findings: Firstly, in our recent study [Citation14] we reported that there may be an insufficient targeted binding of bisphosphonates to sites of high bone turnover in approximately 1/3 of MM and 1/10 of BC patients. Secondly, we observed that if (particularly MM patients) were not treated on a monthly basis, slightly more (but significant) Zol was retained in the skeleton at the next infusion.
What could be the reason that there may be such an insufficient delivery to sites of pathological bone turnover? Delivery occurs through the vasculature and has to be delivered to the bone surface. This may occur through the so-called bone remodeling compartment [Citation21] since this is intimately connected to the vasculature and covers bone remodeling units [Citation22]. Furthermore, the bone remodeling compartment was found to be disrupted in MM patients [Citation23] and thereby the connection to vasculature may also be lost and may explain why we observed an insufficient targeting of bisphosphonate to sites of pathologic bone turnover [Citation14] in 1/3 of the MM patients. More investigations are needed to test this hypothesis.
When comparing the response of bone marker levels to Zol treatment between MM and BC patients we noted that the two disease groups showed a different response. In respect to CTX a strong decline from comparable initial levels were seen in both MM and BC patients. However, after prolonged treatment it was clear that CTX was far stronger suppressed in MM than it was the case in BC patients. At the same time bALP was found to drop very strongly in MM with prolonged treatment while it was only minor in BC patients. In addition the suppression of bALP was found to be dependent on the dosing since those patients that were treated less frequently had higher bALP levels. In contrast, bALP was permanently suppressed no matter if patients were treated every month or every third month. This suggests that Zol suppresses bone formation very strongly at all sites of bone turnover not just those with pathological activity in MM patients but also that this is much less pronounced in BC. This suggests that the risk of inducing “frozen bone” [Citation12,Citation13] in otherwise healthy parts of the bone is more pronounced in MM than BC patients.
Through uni- and multivariate statistics we found that creatinine clearance was significantly reduced in those patients that were treated multiple times with Zol. Although the correlation analyses indicated that this was only significant for MM patients, the multivariate analysis could not rule out that it also accounts for BC patients. Although age and time from diagnosis were found to correlate with creatinine clearance, multivariate statistics showed that the accumulated number of Zol treatments is a strong predictor of creatinine clearance independently of other factors. The results of our prospective study supports previous reports strongly suggesting that prolonged treatment with Zol may compromise kidney function [Citation24,Citation25].
In conclusion, we are faced with the difficult clinical challenge of trading efficient control of bone disease versus preservation of kidney function. In this aspect it is important to consider that we in our present protocol found that Zol dosing has the most pronounced impact on decreasing bone marker levels while it is the accumulated number of Zol treatments which most strongly affect kidney function.
Notice of correction
The version of this article published online ahead of print on 28 Oct 2013 contained an error on page 6. Some parts of the figure legend have been left out. The error has been corrected for this version.
Acknowledgments
We are deeply grateful for the professional, excellent collection and documentation of data according to the GCP guidelines by the project nurses Anne Tørsleff and Annette Rehmeier, and Vibeke Nielsen and Birgit MacDonald for collecting and freezing down most of the urine samples. We wish to thank statistician, René Holst, for statistical advice. We thank Novartis for financial support and interest in the protocol. We would also like to thank the GCP unit of Odense University Hospital for an uncomplicated cooperation. Kent Søe has received partial research funding for the present protocol by Novartis. Torben Plesner has received research funding from Novartis. Erik Jakobsen has been co-investigator on several Novartis trials but has never obtained any financial support. Novartis paid in part the costs of this clinical trial, but had no influence on the study design, interpretation of data or on the decision to submit the manuscript. Novartis merely had the opportunity to comment on the manuscript prior to submission.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Coleman R, Gnant M, Morgan G, Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst 2012;104:1059–67.
- Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): A randomised controlled trial. Lancet 2011;376:1989–99.
- Yan T, Yin W, Zhou Q, Zhou L, Jiang Y, Du Y, et al. The efficacy of zoledronic acid in breast cancer adjuvant therapy: A meta-analysis of randomised controlled trials. Eur J Cancer 2012;48:187–95.
- Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, et al. Guidance on the use of bisphosphonates in solid tumours: Recommendations of an international expert panel. Ann Oncol 2008;19:420–32.
- Guise TA, Brufsky A, Coleman RE. Understanding and optimizing bone health in breast cancer. Curr Med Res Opin 2010;26(Suppl 3):3–20.
- Lund T, Abildgaard N, Delaisse JM, Plesner T. Effect of withdrawal of zoledronic acid treatment on bone remodelling markers in multiple myeloma. Br J Haematol 2010; 151:92–3.
- Richardson PG, Laubach JP, Schlossman RL, Ghobrial IM, Mitsiades CS, Rosenblatt J, et al. The Medical Research Council Myeloma IX trial: The impact on treatment paradigms. Eur J Haematol 2012;88:1–7.
- Terpos E, Sezer O, Croucher PI, Garcia-Sanz R, Boccadoro M, San Miguel J, et al. The use of bisphosphonates in multiple myeloma: Recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol 2009;20:1303–17.
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008; 111:2516–20.
- Chia SK, Speers CH, D’yachkova Y, Kang A, Malfair-Taylor S, Barnett J, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 2007; 110:973–9.
- Giordano SH, Buzdar AU, Smith TL, Kau SW, Yang Y, Hortobagyi GN. Is breast cancer survival improving?. Cancer 2004;100:44–52.
- Abrahamsen B. Bone freezing and bisphosphonates. Maturitas 2011;69:1–2.
- Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CYC. Severely suppressed bone turnover: A potential complication of Alendronate Therapy. J Clin Endocrinol Metab 2005;90:1294–301.
- Soe K, Plesner T, Jakobsen EH, Hansen CT, Jorgensen HB, Delaisse JM. Is retention of zoledronic acid onto bone different in multiple myeloma and breast cancer patients with bone metastasis?. J Bone Miner Res 2013;28:1738–50.
- Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 2011;117:4691–5.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000;92:205–16.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41.
- Hadji P. Managing bone health with zoledronic acid: A review of randomized clinical study results. Climacteric 2011;14:321–32.
- Hadji P, Gnant M, Aapro M, Lipton A, Coleman R. Dosing of zoledronic acid throughout the treatment continuum in breast cancer. Crit Rev Oncol Hematol 2011;79:175–88.
- Lund T, Abildgaard N, Andersen TL, Delaisse JM, Plesner T. Multiple myeloma: Changes in serum C-terminal telopeptide of collagen type I and bone-specific alkaline phosphatase can be used in daily practice to detect imminent osteolysis. Eur J Haematol 2010;84:412–20.
- Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res 2001;16:1575–82.
- Andersen TL, Sondergaard TE, Skorzynska KE, gnaes-Hansen F, Plesner TL, Hauge EM, et al. A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol 2009;174:239–47.
- Andersen TL, Soe K, Sondergaard TE, Plesner T, Delaisse JM. Myeloma cell-induced disruption of bone remodelling compartments leads to osteolytic lesions and generation of osteoclast-myeloma hybrid cells. Br J Haematol 2010; 148:551–61.
- Oh WK, Proctor K, Nakabayashi M, Evan C, Tormey LK, Daskivich T, et al. The risk of renal impairment in hormone-refractory prostate cancer patients with bone metastases treated with zoledronic acid. Cancer 2007;109:1090–6.
- Weide R, Koppler H, Antras L, Smith M, Chang MP, Green J et al. Renal toxicity in patients with multiple myeloma receiving zoledronic acid vs. ibandronate: A retrospective medical records review. J Cancer Res Ther 2010;6:31–5.

