To the Editor,
Treatment options are limited for patients who experience a relapse in glioblastoma (GBM) after initial treatment with surgery, radiotherapy and chemotherapy. The use of anti-angiogenic agents has become part of a salvage-treatment in recurrent GBM in many countries, although an internationally standardised salvage regimen is still lacking. Anti- angiogenic drugs primarily target vascular endothelial growth factors (VEGF) or its receptors. In glioblastoma VEGF is highly expressed, hence, inhibition of VEGF appears to be a rational therapeutic approach. Bevacizumab is a humanised monoclonal antibody specifically inhibiting VEGF, thus preventing the interaction with VEGF receptors on tumour and on vascular endothelial cells. However, anti-angiogenic agents share common adverse effects, including arterial hypertension [Citation1–3]. The causal mechanism behind induced hypertension by anti-angiogenic drugs is still elusive. VEGF upregulates nitric oxide and prostacyclin, leading to vasodilatation, which is counteracted by bevacizumab [Citation4]. Furthermore, bevacizumab counteracts microvascular network formation, which is required for maintenance of normal blood pressure [Citation3]. Blood pressure elevation induced by bevacizumab may predict the efficacy of the drug [Citation5]. In fact, several retrospective series of patients with renal cell carcinoma (n = 53) [Citation6], colorectal carcinoma (n = 39 and n = 181) [Citation7,Citation8] and melanoma (n = 35) [Citation9] have postulated a correlation of blood pressure elevation and better outcome with bevacizumab. However, in a retrospective series of patients with recurrent GBM (n = 51) this observation could not be confirmed [Citation10]. More recently, Lombardi et al. [Citation11] retrospectively analysed 53 patients with recurrent GBM treated with two distinct anti- angiogenic drugs, sorafenib (n = 30) and bevacizumab (n = 23). Twenty patients (38%) developed grades 2–3 hypertension according to common toxicity criteria of adverse events (CTCAE) version 4, indicating a medical intervention. Interestingly hypertension occurred as early as within two months of treatment with bevacizumab. In their study, a significant association was found between hypertension and disease control rate. According to univariate and multivariate analyses, hypertension was related to a longer median survival from anti-angiogenic drug administration, and was reported to be 9.8 versus 4.8 months (p = 0.001; hazard ratio = 3.5, 95% CI 1.6–7.6). The authors postulated that the development of hypertension might be a predictive biomarker in patients with recurrent GBM treated with anti-angiogenic drugs.
Predictive markers for response to anti- angiogenic treatment are urgently needed to guide clinical decision making, to destine therapy towards a well-selected subgroup of patients and to guarantee cost-effectiveness. To compare different study results, definition of hypertension caused by bevacizumab is a key factor. The CTCAE version 4 criteria- and grading system is the most frequent used instrument for toxicity assessment in oncology and thus appropriate for comparison.
We aimed to prospectively investigate the role of hypertension induction as a potential predictive marker for bevacizumab efficacy in recurrent high-grade glioma.
Material and methods
The study was conducted as an open labelled, single-centre prospective cohort study. The study protocol was approved by the local ethics committee (Kantonale Ethikkommission Zürich, EK-1700) and by the Swiss Agency for Therapeutic Products (Swissmedic, 2009DR4247). All participating patients provided signed informed consent. Inclusion criteria were age ≥ 18 years, diagnosis of recurrent anaplastic astrocytoma (WHO Grade III) or glioblastoma (WHO Grade IV) and a planned or established salvage treatment with bevacizumab. Patients with a Karnofsky Performance Status (KPS) of < 60 and patients with prior anti-angiogenic treatment other than bevacizumab were not included in the study. Bevacizumab was administered as an intravenous infusion at our local standard dose of 10 mg/kg every 21 days.
Peripheral blood pressure was measured using Microlife Watch BP® devices (www.watchbp.com, Microlife WatchBP AG, Widnau, Switzerland) prior to, during and after bevacizumab infusion. Patients were in a supine position for at least five minutes before the first blood pressure was taken and during all subsequent blood pressure measurements.
End points
The primary endpoint of the study was to compare overall survival (OS) in patients with and without bevacizumab-associated hypertension, as defined below.
Definition of overall survival from first dose of bevacizumab onwards
OS was defined as time (in days) from the first dose of bevacizumab to death. In patients who were still alive at the end of the study, their OS time was considered as right-censored.
Definition of bevacizumab-associated hypertension
In patients with a BP of < 140 mmHg systolic and < 90 mmHg diastolic before the first administration of bevacizumab (irrespective of antihypertensive treatment), bevacizumab-associated hypertension was defined as a blood pressure of ≥ 140 mmHg systolic and/or ≥ 90 mmHg diastolic during the first three months after the start of bevacizumab. Our definition correlates to an at least grade 2 hypertension according to CTCAE v 4.
In patients with a BP of ≥ 140 mmHg systolic and/or ≥ 90 mmHg diastolic before the first dose of bevacizumab (irrespective of antihypertensive treatment), bevacizumab-associated hypertension was defined as an increase in systolic and/or diastolic blood pressure of > 10 mmHg during the first three months after the start of bevacizumab.
In a few patients who were included in the study at the time of the second or subsequent bevacizumab dose, the BP before the first dose of bevacizumab was retrospectively analysed from the patients` chart.
Statistical analysis
OS from first dose of bevacizumab onwards was compared for the two groups of patients with and without bevacizumab-associated hypertension respectively, using the log rank test. A Cox proportional hazards regression model was fitted, with OS as the dependent variable and bevacizumab- associated hypertension as the determinant of interest. In order to assess the adjusted relationship of bevacizumab-associated hypertension with OS we included the following confounders: age, gender and KPS in the multiple regression model. All statistical analyses were performed with R [Citation12].
Results
Patient and tumour characteristics
Forty patients, 22 males and 18 females, were prospectively included in the study from January 2010 until June 2012. Mean age of the patients was 54.1 ± 9.2 years. Thirty-seven patients had a glioblastoma and three an anaplastic astrocytoma. Karnofsky Performance Status at baseline was 75.8 ± 13.7. Eight patients have had a history of hypertension prior to study entry, half of them were treated with an antihypertensive agent. Eighty-three percent of patients were on steroids prior to treatment with bevacizumab (Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.852240). Follow-up of patient survival was performed until April 2013.
Table I. Results of the multiple Cox proportional hazards model for overall survival.
Bevacizumab-associated hypertension
Seventeen of 40 (42%) patients developed bevacizumab-associated hypertension within three months after the first dose of bevacizumab. Ten patients developed grade 2 and seven patients developed grade 3 CTCAE v 4 hypertension.
Overall survival after first dose of bevacizumab
Median OS was 275 (117–390) days or 9.0 months in patients without bevacizumab-associated hypertension and 177 (130–222) days or 5.8 months in patients with bevacizumab-associated hypertension. Four patients were right-censored, because they were still alive at the time of the last follow-up; one patient was still on bevacizumab therapy at last follow-up.
shows OS from first dose of bevacizumab in patients with and without bevacizumab-associated hypertension. No statistically significant difference in OS was found between the two groups with the log rank test (p = 0.16). Censored patients are depicted with vertical lines.
Figure 1. Overall survival after first dose of bevacizumab in patients with (solid lines) and without (dotted lines) bevacizumab-associated hypertension (p-value log rank test = 0.16). Bevacizumab associated hypertension was defined as 1) blood pressure of ≥ 140 mmHg systolic and/or ≥ 90 mmHg diastolic during the first 3 months after the start of bevacizumab in patients without prior hypertension, or 2) increase in systolic and/or diastolic blood pressure of > 10 mmHg during the first 3 months after the start of bevacizumab in patients with pre-existing hypertension. Censored patients (n = 4).
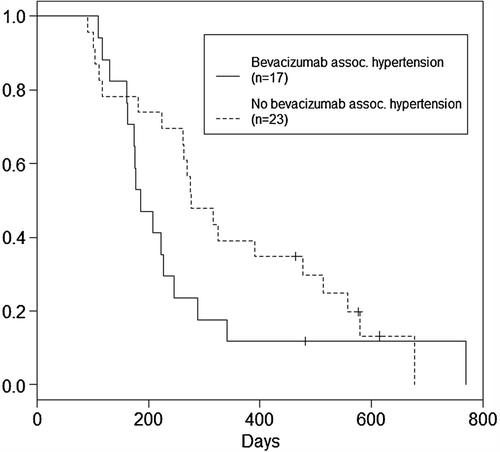
The Cox proportional hazards model was fitted to OS to quantify the adjusted effect of bevacizumab-associated hypertension, accounting for a set of confounders. Again the development of bevacizumab-associated hypertension did not significantly affect OS, although the estimated hazard ratio was equal to 1.72 (p = 0.14). Results can be found in .
Capture of salvage treatment after bevacizumab
Median survival after bevacizumab failure was 78 days (range 14–401) for the whole group. Twenty-one patients received further chemotherapy, including drugs such as temozolamide, carboplatin, lomustine and everolimus. Their median survival was 84 days (range 40–390). Eighteen patients received no further treatment. Their median survival was 58 days (14–401).
Eleven of 17 patients that developed bevacizumab-associated hypertension received further salvage therapy whereas 10 of 23 patients without bevacizumab-associated hypertension received further therapy.
Safety
Twelve patients experienced serious adverse events, most of them not related or unlikely to be related to treatment with bevacizumab (e.g. progression of neurological symptoms, seizures). There was only one serious adverse event (pulmonary embolism) that was possibly related to treatment with bevacizumab.
Discussion
Results from phase II studies are inconsistent respective to hypertension as a predictor for clinical efficacy of bevacizumab in advanced cancer. Our prospective study provides evidence that bevacizumab monotherapy is effective in recurrent high-grade glioma independent of developing hypertension. According to our definition, corresponding to at least grade 2 (CTCAE v 4) hypertension, 42% of our patients developed early hypertension, i.e. three months after start of bevacizumab. On purpose we did select a low threshold for our definition of bevacizumab-induced HT in order to capture even small differences in blood pressure. The proportion of patients with bevacizumab-induced HT in our cohort is in line with results from larger studies, e.g. the BRAIN study that led to the FDA approval of bevacizumab in recurrent GBM, where 35% induced hypertension of all grades was reported [Citation13]. A large double blinded, placebo controlled Phase III trial, attempting to address the benefit of bevacizumab in newly diagnosed GBM alongside the standard of care with radiation and chemotherapy reported 37.5% hypertension of all grades in the bevacizumab arm [Citation14].
As mentioned in the introduction, 53 patients suffering from recurrent GBM treated by Lombardi et al. with sorafenib (n = 30) and bevacizumab (n = 23) developed grade 2–3 hypertension in 38% (CTCAE v 4) [Citation11]. A correlation between the onset of hypertension and benefit from bevacizumab was postulated, however the authors called for a prospective trial to validate their findings [Citation11]. Apart from being a single centre, retrospective analysis, more than half of the patients were treated with sorafenib, which per se might result in a different outcome in recurrent GBM. In contrast to the results by Lombardi, a retrospective series by Wick et al. with 51 patients treated with bevacizumab for recurrent GBM could not confirm an association between outcome and development of hypertension [Citation10].
We are aware of limitations of our study. The number of patients is small and treatment after failure of bevacizumab might have slightly influenced outcome. Twenty-one patients (50%) who received additional salvage treatment lived a median of 30 days longer than those without salvage therapy. However, patients with salvage treatment after bevacizumab were well balanced in both groups and patients surviving more than one year after cessation of bevacizumab were also found in both groups. Antihypertensive drugs such as angiotensin converting enzyme inhibitors and angiotensin receptor blockers appear to have beneficial effects on tumour progression, vascularisation and metastasis [Citation15,Citation16]. However, the proportion of patients on these drugs in our study was negligible.
Conclusions
Our prospectively collected data do not support the notion of induced hypertension as a predictor of clinical benefit from anti-angiogenic drugs such as bevacizumab, at least not in high-grade gliomas.
Ongoing and future trials should focus on factors that may drive bevacizumab activity. Tumour tissue, blood- and/or urine-derived biomarkers as well as other tools to investigate bevacizumab activity including imaging will hopefully accurately predict and monitor effects of anti-angiogenic therapy in cancer.
Supplementary Table I
Download PDF (489.9 KB)Declaration of interest: CW, UH, RK, EB and LZ declare no conflicts of interest. SH received honoraria from Roche for advisory board participation and lecturing.
References
- Mir O, Ropert S, Alexandre J, Goldwasser F. Hypertension as a surrogate marker for the activity of anti-VEGF agents. Ann Oncol 2009;20:967–70.
- Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, et al. Hypertension as a biomarker of efficacy in patients with metastastic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2011;103:763–73.
- Humar R, Zimmerli L, Battegay E. Angiogenesis and hypertension: An update. J Hum Hypertens 2009;23:773–82.
- Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 2007;96: 1788–95.
- Jubb A, Harris A. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol 2010;11:1172–83.
- Bono P, Elfving H, Utriainen T, Osterlund P, Saarto T, Alanko T, et al. Hypertension and clinical benefit of bevacizumab in the treatment of advanced renal cell carcinoma. Ann Oncol 2009;20:393–4.
- Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol 2009;20:227–30.
- Tahover E, Uziely B, Salah A, Temper M, Peretz T, Hubert A. Hypertension as a predictive biomarker in bevacizumab treatment for colorectal cancer patients. Med Oncol 2013;30:327.
- Schuster C, Eikesdal HP, Puntervoll H, Geisler J, Geisler S, Heinrich D, et al. Clinical efficacy and safety of bevacizumab monotherapy in patients with metastatic melanoma: Predictive importance of induced early hypertension. PLos One 2012;7:e38364.
- Wick A, Schäfer N, Dörner N, Schemmer D, Platten M, Bendszus M, et al. Arterial hypertension and bevacizumab treatment in glioblastoma: No correlation with clinical outcome. J Neurooncol 2010;97:157–8.
- Lombardi G, Zustovich F, Farina P, Fiduccia P, Della Puppa A, Polo V, et al. Hypertension as a biomarker in patients with recurrent glioblastoma treated with antiangiogenic drugs: A single-center experience and a critical review of the literature. Anticancer Drugs 2013;24:90–7.
- Development Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, Available from: http://www.R-project.org/ [cited 2013 Oct 29].
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27: 4733–40.
- Wick W, Cloughesy TF, Nishikawa R, Mason W, Saran F, Henriksson R, et al. Tumor response based on adapted Macdonald criteria and assessment of pseudoprogression in the phase III AVAglio trial of bevacizumab plus temozolomide plus radiotherapy in newly diagnosed glioblastoma patients. Proc Am Soc Clin Oncol 2013;31 (Suppl Abstract 2002).
- Deshayes F, Nahmias C. Angiotensin receptors: A new role in cancer?Trends Endocrinol Metab 2005;16:293–9.
- Arafat HA, Gong Q, Chipitsyna G, Rizvi A, Saa CT, Yeo CJ, et al. Antihypertensives as novel antineoplastics: Angiotensin-I-converting enzyme inhibitors and angiotensin II type 1 receptor blockers in pancreatic ductal adenocarcinoma. J Am Coll Surg 2007;204:996–1006.

