To the Editor,
Treatment outcomes for prostate cancer radiotherapy improve with increased dose delivered to the prostate [Citation1]. To enable dose escalation to the prostate without increasing treatment toxicity requires reducing the planning target volume (PTV) margins which allow for prostate movement within the pelvis. A major cause of intrafraction prostate motion is due to changes in the rectal volume which lies adjacent to the prostate [Citation2,Citation3]. A potential method to reduce prostate motion within the pelvis is to alter the dietary intake by reducing fibre and gas producing foods of patients who receive radiotherapy.
Previously reported studies provide inconclusive evidence regarding the effectiveness of diet interventions. A study using magnesium oxide laxative with an antiflatulent diet reported a trend of reduced prostate motion [Citation4], while other work using a similar intervention reported no impact on prostate motion [Citation5,Citation6].
The current study investigated an antiflatulent diet combined with psyllium husk (psyllium) during radiotherapy. The study hypothesis was that the diet intervention can minimise bowel gas production to levels absorbable within the intestine and allow patients to present for treatment with an empty rectum. This pilot study was conducted to assess feasibility and inform the sample size for a definitively powered study to investigate if a diet intervention would reduce rectal volume variation when compared to standard radiotherapy for prostate cancer.
Material and methods
This study was a randomised, controlled pilot study with a pragmatic sample of 15 participants in each arm to generate activity data. Following Human Ethical Review approval, 30 participants were enrolled at Peter MacCallum Cancer Centre Bendigo, Australia between February 2010 and July 2011. Eligible participants were 50 years of age or older receiving external beam radiotherapy (EBRT) to the intact prostate, TNM stages T1–T3b. All participants received implanted prostate fiducials with daily imaging as part of our standard care [Citation7], had ECOG performance status 0–2 and provided written informed consent. Those who gave consent were randomised by telephone using a computer-generated varied size block randomisation. The participants and investigators were not blinded to their randomisation assignment, although blinding did occur during organ contouring as described below.
Treatment delivery and interventions
All participants were prescribed radiotherapy of 74–78 Gy in 2 Gy fractions. Planning and treatment was in the supine position with immobilisation using a Combifix (CIVCO Medical Solutions, Kalona, USA). Pre-treatment kilovoltage (kV) paired images were acquired with a 0 mm tolerance online correction to gold fiducials applied each day before treatment. Cone-beam CT (CBCT) images were acquired at the end of treatment for fractions 1–5 and then every second fraction. Missed CBCT acquisitions due to mechanical or system failure were not repeated, however, CBCTs not acquired due to micturition urge were repeated at the next fraction.
Standard therapy (ST) bladder and bowel preparation instructions were to consume 750 ml of water 30 minutes before treatment and to take 5 g/d Fybogel (Reckitt Benckiser, Slough, UK) if required to promote regular bowel motions.
The diet intervention (DI) involved the consumption of psyllium as a bulk forming laxative. Psyllium dose was 20 g/d and instructions to consume at least two litres of water per day to avoid dehydration or constipation. The antiflatulent diet developed for this project is outlined in Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2013.854927). Recommendations were to avoid excessive dairy intake, hot and spicy foods, the stems and skins of fruits and vegetables, and to eat cooked vegetables warm. Reducing lipid intake, which delays gas transit, was recommended [Citation8]. Instructions were given to reduce aerophagia and increase exercise to promote frequent bowel motions [Citation4]. Patients were asked to empty bowel and bladder 50 minutes before treatment, to consume 750 ml of water from 45 until 30 minutes before treatment and to maintain bladder filling until the end of treatment. If feeling of gas present in bowel, patients were to expel the gas if possible. The intervention also advised to avoid caffeine intake in the two hours prior to treatment.
Table I. Demographic characteristics of prostate cancer patients participating in a diet intervention and bowel motion compliance study (total N = 30).
All participants completed a daily diet diary from two weeks prior to their CT planning appointment until the end of treatment following the recommendations outlined in a previous study [Citation9]. The DI followed the intervention for the same duration. DI participants were provided with psyllium and diet intervention guidelines.
Contouring
De-identified datasets were contoured on FOCAL 4.62 (Elekta, Stockholm, Sweden) by one investigator (DJ) who was blinded to the randomisation assignment. The rectum was contoured as the external surface of the rectum from the nearest slice to 9 mm inferior to the top (superior border) and to the nearest slice to 9 mm superior to the bottom (inferior border) of the treatment field. Contouring this way minimised including the sigmoid colon and anal canal with prostate motion. The prostate was contoured as the external surface of the prostate from the base to the apex. Seminal vesicles were not contoured for the study.
Data collection and statistical methods
Rectal volumes were recorded from all CBCTs contours using XiO 4.62 (Elekta). The CBCT intra-patient rectal volume standard deviation for each patient was calculated as a measure of their rectal volume variability. On each scan at the centre and superior slices of the prostate the rectal filling was assessed by the principal investigator and was given a score of empty, gas, moving gas and/or faeces using a similar criteria to Smitmans et al. [Citation4]. ‘Empty’ was defined as a measurable lumen diameter of less than 1 cm in the axial plane or outer rectal surface of less than 3 cm in diameter.
Per-protocol analysis was performed, withdrawn patients were excluded from the analysis. All analyses were performed using R software version 2.15.1 (www.r-project.org/). All tests were two-tailed with an alpha of 0.05. The Welch's t-test was used to assess the difference between arms in within patient rectal volume variability due to larger between patient variation in the standard therapy arm. Pearson's χ2-test was used to assess the difference between arms in terms of rectal filling. The assumption was made that each participant's rectal filling at a given fraction was independent to their rectal filling at subsequent fractions.
Results
Over 16 months 56 patients were approached for participation, demographics for the 30 participants are in . Eight patients withdrew during the study. While the overall attrition was 26.6%, only three patients withdrew potentially related to the diet intervention. One was due to dehydration, one due to diabetes diagnosis before starting the diet, which was not stabilised with medication and the last due to constipation issues which was complicated by his hypothyroidism.
Rectal volume variability
Usable CBCT datasets were available for 435 fractions (ST = 238, DI = 197) with a median (range) of 20 (16–22) CBCT per patient. The mean intra- patient rectal volume variability for ST was 15.8 cm3 (95% CI 10.9–20.7) and for DI it was 11.8 cm3 (95% CI 9.1–14.5). This difference did not reach statistical significance with an estimate of the mean difference 4.0 (95% CI -1.4–9.3, p = 0.133). Significance was not reached due to the small number of samples, however, there appears to be activity with the intervention ().
Figure 1. Boxplot displaying the per-patient rectal volume standard deviation – a measure of rectal volume variability, grouped per randomisation. The bar represents the median rectal volume standard deviation per randomisation; the box represents the inter-quartile range and the whiskers represent the 95 percentile.
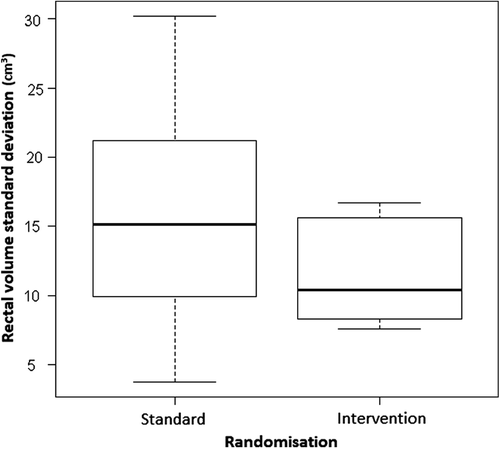
Using the data available, a conservative sample size requires 40 patients in each arm to detect a difference of 4 cm3 in the average rectal standard deviation between the two study arms. This sample gives a power of > 0.80 at the significance level of 0.05. A sample size of approximately 50 patients per arm would be required to allow for a 20% withdrawal rate.
Rectal filling
Overall, no relationship between diet and superior rectal filling was demonstrated (). At the centre rectal filling a relationship was found between diet intake and a rectum that was empty, filled with gas or faeces, with patients in the DI arm more likely to have an empty rectum, absent of gas and faeces. No relationship was demonstrated between diet intervention and moving gas.
Table II. Relationship between rectal filling at superior and centre slices of the rectum to randomisation of standard therapy or diet intervention.
Discussion and Conclusion
Our results demonstrate that a diet intervention appears to reduce rectal volume variability when compared with standard therapy. These results provide preliminary data for a sample size of 100 participants for a future study.
A previous diet intervention study of an antiflatulent diet with magnesium oxide was published by Smitmans and colleagues [Citation4]. Their study of CBCT from 26 patients who followed the intervention compared to 23 historic datasets suggested a trend of reduced prostate motion, indicating activity, however, their results were not statistically significant. At the prostate level they found a significant reduction of gas, moving gas and faeces in the intervention arm, which agreed with our results for gas and faeces for the centre of the prostate. However, our study also assessed superior prostate level which did not demonstrate any significant changes in rectal filling, potentially due to greater variation in rectal filling at that level.
A subsequent study by Lips et al. [Citation6] compared the intrafraction motion of 105 patients following the antiflatulent intervention described by Smitmans and colleagues to 739 patients without intervention and did not find reduced intrafraction prostate motion. A critical difference between the two studies was that Lips did not include a laxative in their intervention, which may have impacted on their results.
In another study using antiflatulent diet and milk of magnesia as the DI they used 42 patients with intra-patient controls to test the intervention [Citation5]. A pre-intervention cine-MRI was taken to observe prostate motion and rectal filling, followed by a cine-MRI with diet intervention at the time of planning CT and one during the course of treatment. They found that moving gas was the main cause of prostate intrafraction motion. However, because they asked their participants to void their bowel immediately before each scan, they did not detect a decrease in intrafraction prostate motion with the intervention.
A different intervention approach was taken by McNair and colleagues [Citation10]. Their protocol required a baseline assessment of dietary intake followed by a fibre and fluids prescription based on diet analysis. They also scheduled radiotherapy appointments within two hours of the planning CT appointment time. Their results, from 22 patients found that a small modification of fibre intake resulted in no improvement in rectal volume consistency, potentially due to the small change in diet and the inclusion of fibre which may cause bowel gas.
In conclusion a diet intervention of an antiflatulent diet supplemented with psyllium husk appears to offer a more consistent rectal volume when compared to no intervention. The findings reported here support further investigation of efficacy of a DI in a larger cohort.
Supplementary Table I
Download PDF (414.2 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: A meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys 2009;74:1405–18.
- Ghilezan MJ, Jaffray DA, Siewerdsen JH, Van Herk M, Shetty A, Sharpe MB, et al. Prostate gland motion assessed with cine-magnetic resonance imaging (cine-MRI). Int J Radiat Oncol Biol Phys 2005;62:406–17.
- Padhani AR, Khoo VS, Suckling J, Husband JE, Leach MO, Dearnaley DP. Evaluating the effect of rectal distension and rectal movement on prostate gland position using cine MRI. Int J Radiat Oncol Biol Phys 1999;44:525–33.
- Smitsmans MHP, Pos FJ, de Bois J, Heemsbergen WD, Sonke J-J, Lebesque JV, et al. The influence of a dietary protocol on cone beam ct-guided radiotherapy for prostate cancer patients. Int J Radiat Oncol Biol Phys 2008;71: 1279–86.
- Nichol AM, Warde PR, Lockwood GA, Kirilova AK, Bayley A, Bristow R, et al. A cinematic magnetic resonance imaging study of milk of magnesia laxative and an antiflatulent diet to reduce intrafraction prostate motion. Int J Radiat Oncol Biol Phys 2010;77:1072–8.
- Lips IM, Kotte ANTJ, van Gils CH, van Leerdam ME, van der Heide UA, van Vulpen M. Influence of antiflatulent dietary advice on intrafraction motion for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys 2011;81: e401–6.
- Thompson A, Fox C, Foroudi F, Styles C, Tai KH, Owen R, et al. Planning and implementing an implanted fiducial programme for prostate cancer radiation therapy. J Med Imaging Radiat Oncol 2008;52:419–24.
- Azpiroz F. Intestinal gas dynamics: Mechanisms and clinical relevance. Gut 2005;54:893–5.
- Oates R, McPhee N, Joon ML, Schneider-Kolsky M, Kron T. Recording a patient diet over the radical course of radiotherapy for prostate cancer using a diet diary: A feasibility study. J Radiother Pract 2013;12:18–25.
- McNair HA, Wedlake L, McVey GP, Thomas K, Andreyev J, Dearnaley DP. Can diet combined with treatment scheduling achieve consistency of rectal filling in patients receiving radiotherapy to the prostate?Radiother Oncol 2011;101:471–8.

