Abstract
Background. Few studies to date have described the clinical features of malignant melanoma in young adulthood. Also, little is known about patterns of care in young patients. We examined and compared clinical characteristics, management and survival between young adult (15–39 years) and older adult melanoma patients in Central Sweden. Material and methods. Patients diagnosed with invasive malignant melanoma between 1997 and 2011 were identified in the Regional Quality Register of Cutaneous Malignant Melanoma in Central Sweden, a population-based register covering a source population of about two million. Data on clinical characteristics, management and survival were retrieved and compared according to age at diagnosis. Results. Of 5915 patients included in the study, 584 (9.9%) were between 15 and 39 years of age at diagnosis. Compared with older patients, young adult patients were more likely to be female, with higher proportions of thin, non-ulcerated melanomas, superficial spreading melanoma and melanomas located on the lower extremity. Young adults had shorter waiting times for surgical procedures and a higher proportion received surgical treatment according to guidelines. Overall, young patients had better relative survival than older patients. Age-related survival differences varied by stage of disease at diagnosis, and were most prominent in stage II disease. Conclusion. The observed differences in clinical characteristics, management and survival between young adult and older melanoma patients call for an improved understanding of not only disease etiology but also factors driving management decisions. A better understanding of these differences may help improve care and prognosis for melanoma patients of all ages.
Malignant melanoma of the skin is one of the most common malignancies in adolescents and young adults in Europe, Northern America and Australia/New Zealand [Citation1]. In Sweden, it is the second most common cancer type in adolescents and young adults aged 15–39 years (hereafter referred to as young adults), accounting for 16% of all cancers in this age group [Citation2]. The incidence of melanoma in Sweden has increased dramatically in recent decades. Although the highest increase has been seen in older adults, the incidence of melanoma among young adults has more than tripled since the 1960s [Citation3]. A similar pattern has been observed in other European countries [Citation3,Citation4] and was recently reported for the US [Citation5,Citation6].
Malignancies in young adulthood, including melanoma, have attracted less attention than cancers in childhood and later adulthood. Although there are many knowledge gaps in this research area, previous studies suggest that cancers in young adulthood are different from malignancies occurring later in life in terms of biology and prognosis [Citation7]. Only a few US-based studies have examined the specific features of melanoma in young adulthood [Citation6,Citation8,Citation9]. Results to date indicate that younger patients diagnosed with melanoma are more likely to be female [Citation8,Citation10], more frequently have melanomas arising on the lower extremity and less frequently on the head and neck [Citation10,Citation11] and present with thinner lesions [Citation8,Citation10,Citation11]. Despite a higher incidence of sentinel node metastasis [Citation8], young age has been associated with a better prognosis [Citation8,Citation10]. Also, some age-related variations in management and treatment have been reported [Citation12], however no previous study has addressed aspects of disease management in young adulthood.
A better understanding of the occurrence, management and prognosis of melanoma in young adulthood is important for both improved treatment and outcome. The aim of the present study was to examine and compare clinical characteristics, management, and survival between young adult and older adult melanoma patients identified in a regional melanoma register in Central Sweden.
Material and methods
Data collection
The present population-based cohort study included all patients aged 15 and over with invasive malignant melanoma, registered in the Regional Quality Register of Cutaneous Malignant Melanoma of the Uppsala/Örebro Health Care Region between 1997 and 2011. The register was established to monitor and improve the quality of care, and includes detailed information about diagnosis, tumor characteristics, clinical stage and surgical procedures. The register covers a source population of about two million, representing 21% of Sweden's total population. Compared with the Swedish Cancer Register to which reporting is mandated, the completeness of the register exceeds 95% [Citation13]. Information on vital status is obtained by updates from the National Population Register.
In total, we identified 7808 patients aged 15 and over in the register between 1 January 1997 and 31 December 2011. The analysis was restricted to 5915 cases with invasive melanoma, excluding 1859 cases with in situ melanoma and 34 cases with missing information on type of melanoma.
Information on patient and tumor characteristics included sex, age at diagnosis, date of diagnosis, anatomic location of the tumor, histologic subtype, Clark level of invasion, Breslow thickness, presence of ulceration and regression, and regional and distant metastasis. Data were available on date of first visit (for patients diagnosed after 1 January 2009), date and type of surgery, institution where surgery was performed, excision margins and tumor growth at the line of resection.
Assessment of stage was performed according to the 6th version of the American Joint Committee on Cancer's melanoma classification system [Citation14], with the following criteria: stage I (T1a/T1b/T2a N0 M0), stage II (T2b/T3a/T3b/T4a/T4b N0 M0), stage III (Any T N1/N2/N3 M0) and stage IV (Any T Any N Any M1). Patients with unclassifiable disease stage due to missing data were categorized as having stage not available. Staging of regional disease was primarily based on pathological assessment, or in the absence of lymph node surgery (generally not practiced before 2003), on clinical assessment. In national guidelines [Citation15], sentinel node evaluation is recommended for invasive melanomas thicker than 1.0 mm or in the presence of ulceration or Clark level IV–V.
Surgical excision margins were categorized according to recommendations in national guidelines [Citation15]: 10 mm for invasive melanomas thinner than or equal to 1.0 mm, and 20 mm for invasive melanomas thicker than 1.0 mm. Surgeries with a total margin greater than or equal to these recommendations were categorized as adherent to the guidelines.
Statistical methods
Differences in patient and tumor characteristics, management and surgical procedures between age groups (15–39 years, 40–64 years and ≥ 65 years) were compared using the χ2-test for categorical variables and the Kruskal-Wallis test for variables presented as medians. Binary logistic regression models were used to model the association between age group and having surgery adherent to guidelines, adjusted for potential confounders.
Survival time was calculated from date of diagnosis to date of death, emigration or end of follow-up (8 December 2012), whichever came first. Survival experience by age groups was described using relative survival, a measure of excess mortality associated with the diagnosis of cancer, irrespective of whether the cancer is a direct or indirect cause of death [Citation16]. Relative survival was calculated as the ratio of the observed survival in the study population compared to the expected survival of the general population, standardized for calendar year, age and sex. To estimate expected survival in the present study, life-tables of the general population were retrieved from Statistics Sweden. Relative survival rates with 95% confidence intervals (CI) were generated using the Hakulinen estimates of expected survival [Citation17]. Poisson regression models were used to compare the relative survival between age groups, adjusted for potential confounders. Differences between groups are reported as relative excess risk of death (RER) with 95% CI.
All tests were two-tailed and p-values < 0.05 were considered statistically significant. Analyses were carried out using STATA version 11 (STATA Corp., Texas, USA). This study was approved by the Ethical Review Board in Stockholm (dnr 2012/2203-31/5).
Results
Clinical characteristics
Of a total of 5915 patients, 584 (9.9%) were between 15 and 39 years of age at diagnosis. For all characteristics except regression phenomena, significant differences between age groups were found (). The proportion of women was highest in the youngest age group (64.4%), and decreased with increasing age to 45.5% in the oldest age group. Younger patients had more melanomas diagnosed on the lower extremity (33.7% vs. 18.1% in the oldest age group) and a lower proportion of melanomas located in the head and neck region (8.9% vs. 18.9%). Young patients more frequently had superficial spreading melanoma (78.8% vs. 56.3%) and less often nodular melanoma (14.9% vs. 28.1%).
Table I. Clinical characteristics of young adult melanoma patients aged 15–39 years diagnosed between 1997 and 2011 in the Regional Quality Register of Cutaneous Malignant Melanoma of the Uppsala/Örebro Health Care Region, in comparison with older age groups.
In comparison with the oldest age group, young patients more frequently presented with stage I (80.6% vs. 54.8%), but less often with stage II (13.1% vs. 38.0%) and stage IV (0.2% vs. 1.7%) disease. Young patients had a higher proportion of thin (≤ 1.0 mm) tumors (64.9% vs. 40.4%) and lower proportions of tumors with Clark level IV and V. Furthermore, young patients presented less often with ulcerated tumors (12.8% vs. 31.3%).
Management
The majority of patients underwent excision and primary suture; the proportion was 94.5% among young patients compared with 85.9% among the oldest patients (). Young patients less frequently visited a hospital for their primary surgery (46.6% vs. 63.9%), more frequently had surgical procedures without tumor growth at the line of resection (90.0% vs. 84.8%) and more frequently had surgical procedures adherent to excision guidelines (89.3% vs. 79.1%). The likelihood of having a surgical procedure adherent to excision guidelines was two-fold higher in the youngest age group compared with the oldest (odds ratio 2.20, 95% CI 1.56–3.10). Following adjustment for sex, anatomic location, Breslow thickness and calendar period of diagnosis, the corresponding odds ratio was 1.52 (95% CI 1.06–2.19) (data not presented).
Table II. Management and surgical treatment of young adult melanoma patients aged 15–39 years diagnosed between 1997 and 2011 in the Regional Quality Register of Cutaneous Malignant Melanoma of the Uppsala/Örebro Health Care Region, in comparison with older age groups.
The proportion of patients with a record of sentinel lymph node biopsy was lowest in the oldest age group (16.9%), compared with 21.2% in the youngest age group and 24.4% in the middle age group (). No statistically significant differences between age groups were found for having a biopsy-positive sentinel lymph node.
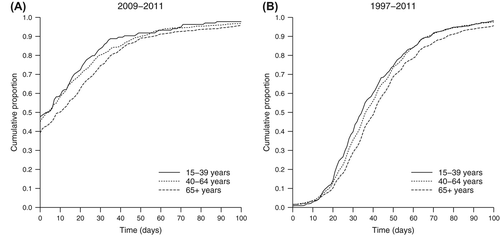
The waiting time between first medical visit and primary surgery, available for patients diagnosed between 2009 and 2011, was shortest among young patients [median 4 days, interquartile range (IQR) 0–22 days] and longest among old patients (median 10 days, IQR 0–31 days, p = 0.009) (). Among young adults, 47.8% underwent primary surgery on the same day as the first visit and 83.6% within the first month. The time interval between primary and extended surgery, available for the whole study period, increased from a median of 35 days (IQR 24–51 days) in the youngest age group to 41 days (IQR 29–56 days) in the oldest (p < 0.001) (). Among young adults, 84.1% underwent the extended surgery within two months and 96.2% within three months.
Figure 1. Surgical time intervals for young adult melanoma patients aged 15–39 years in the Regional Quality Register of Cutaneous Malignant Melanoma of the Uppsala/Örebro Health Care Region, in comparison with older age groups. (A) Time between first visit and primary surgery (data available for patients diagnosed between 2009 and 2011, n = 1816). (B) Time between primary and extended surgery (data available for patients diagnosed between 1997 and 2011, n = 6227).
Survival
All 5915 patients were included in the survival analysis. Six patients had emigrated and were censored on the date of emigration. A total of 1670 deaths occurred during a median follow-up of 4.9 years (range 0.0–15.9 years). Overall, young patients had better relative survival than older patients; the five-year relative survival was 94.3% for age group 15–39, 90.4% for age group 40–64 and 84.1% for age group ≥ 65 (). Within the young adult group, the five-year relative survival was 94.6% in women and 93.7% in men (data not presented).
Figure 2. Cumulative relative survival in young adult melanoma patients aged 15–39 years diagnosed between 1997 and 2011 in the Regional Quality Register of Cutaneous Malignant Melanoma of the Uppsala/Örebro Health Care Region, in comparison with older age groups and according to stage at diagnosis.
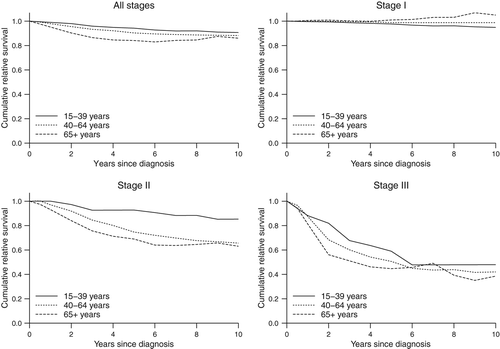
Survival differences between age groups varied by stage of disease at diagnosis (). In stage I disease, the five-year relative survival was 97.7% for the youngest age group, 98.8% for the middle age group and 101.0% for the oldest age group. In stage II disease, the corresponding relative survival was 92.8%, 74.8% and 69.0%, respectively, and in stage III disease 59.0%, 50.6% and 44.7%, respectively.
shows the prognostic role of young age in stages II and III disease using Poisson regression analysis. In univariable analysis for stage II disease, young adults had a significantly lower RER (relative excess risk of death) compared with the oldest age group (RER 0.24, 95% CI 0.12–0.51). Following adjustment for sex, anatomic location, Breslow thickness, ulceration and calendar year of diagnosis, young age remained associated with a lower excess risk (RER 0.31, 95% CI 0.15–0.66). In stage III disease, there was some evidence of a lower excess risk for younger patients, albeit not statistically significant either in univariable analysis (RER 0.63, 95% CI 0.35–1.14) or after adjustment for sex, anatomic location, Breslow thickness, ulceration and calendar year of diagnosis (RER 0.84, 95% CI 0.41–1.71).
Table III. Relative excess risk of death in patients with malignant melanoma diagnosed between 1997 and 2011 in the Regional Quality Register of Cutaneous Malignant Melanoma of the Uppsala/Örebro Health Care Region, according to stage at diagnosis.
Discussion
To our knowledge, this is the first study that has addressed aspects of management of malignant melanoma diagnosed in young adulthood. We found that young adult patients aged 15–39 years had a more favorable distribution of clinical characteristics and underwent surgical procedures with better adherence to guidelines and shorter waiting times compared with older patients. Relative survival was better among young adults, but age-related survival differences varied by stage of disease at diagnosis and were most prominent in stage II disease.
In agreement with previous studies [Citation8,Citation11], including one from the Swedish context [Citation10], we found that young patients are more likely than older patients to be female, and have higher proportions of thin, non-ulcerated melanomas, superficial spreading melanoma and melanomas located on the lower extremity. The female predominance among young adults and the subsequent shift to male predominance in older ages is also seen for other invasive cancers, indicating that sex hormones may play a role in carcinogenesis [Citation7]. It has also been hypothesized that differences in sun exposure during adolescence or early adulthood might be involved in the female predominance seen among young adult patients [Citation18]. Melanomas of different anatomical locations have been suggested to have different causal pathways [Citation19], possibly indicating that the observed age-related differences in location reflect differences in etiology.
In the present study, nearly 90% of young adult patients underwent surgical procedures adherent to excision guidelines. Corroborating reports from France and Germany [Citation20,Citation21], adherence was lowest for the oldest patients. This age-related variation in care is unlikely to be completely explained by other age-related differences such as Breslow thickness and anatomic location, since the association remained in the multivariable analysis, in agreement with findings by Grange et al. [Citation20]. Possible explanations may include that surgeons pay greater attention to recommended margins when managing young patients or that comorbidities among the elderly influence type of surgery and excision margins.
In contrast to surgical excision margins, there is no national consensus on how soon surgical treatment should take place. In the present study, nearly half of young adults underwent primary surgery on the first visit and over 80% within the first month. Compared with older patients the youngest age group had the shortest waiting times, for both the primary and the extended surgery. The literature for surgical time intervals is scarce; two studies addressing delays in the surgical management of melanoma found that old age was associated with longer waiting times between diagnostic biopsy and wide local excision [Citation22,Citation23]. Possible explanations include more complex surgical procedures among elderly due to advanced lesions and a higher prevalence of lesions located on surgically demanding areas (i.e. head and neck).
We found that the survival benefit in young adults was mainly driven by better relative survival in stage II disease, independent of other important prognostic factors such as sex, Breslow thickness and ulceration. Kemeny et al. [Citation24] reported similar relative survival patterns in a large cohort of patients diagnosed in the late 1980s; stage II was the only stage at which both men and women aged 45 and under had higher five-year relative survival rates than patients aged 55 and over. Despite having a generally better prognosis than older adults, our cohort of young adult patients diagnosed with more advanced disease had a five-year relative survival below 60%.
The reasons for the survival advantage observed in young melanoma patients remains incompletely understood. Since age has been found to be an independent prognostic factor in several studies [Citation25], the more favorable distribution of prognostic factors among younger patients is unlikely to fully explain the survival benefit. It has been hypothesized that immunological factors negatively influence survival among the elderly [Citation25]. As observed in the present study and in a recent review [Citation12], elderly melanoma patients are often undertreated. However, earlier findings indicate that minor surgical time delays [Citation22,Citation23] and non-compliance with excision guidelines [Citation26] do not affect survival.
Differences in stage-specific survival between age groups can also reflect differences in diagnostic intensity. In line with earlier results [Citation27], we found some evidence of underuse of sentinel node biopsy in elderly, indicating that a proportion of elderly patients with nodal disease might be misclassified as having early stage disease. As a consequence, survival in early stages might be underestimated.
Our study's strengths include the use of a population-based register with detailed data available also on patterns of care, covering nearly all melanoma cases in Central Sweden and with virtually complete follow-up. Limitations include incomplete reporting on some clinical and surgical variables. However, we could not find an age-related pattern of missing data and we have no reason to believe that reporting to the register is related to the age of the patient. In all age groups, we expect some missed cases of nodal disease due to underreporting of sentinel node surgery and due the lack of lymph node surgery in the earlier calendar years covered by the study. In addition, results for subgroups with a small number of patients should be interpreted with caution.
Overall survival cannot be used to fairly compare patients of different age groups, and since information on cause of death was unavailable, relative survival was used to describe survival. Relative survival is usually preferred over cause-specific survival in population-based registers [Citation16], but the reliability of relative survival estimates depends upon the comparability of the cancer group and the external comparison group [Citation28]. Studies have shown that high socioeconomic status is associated with both better survival [Citation29] and a diagnosis of melanoma [Citation30], indicating that relative survival might be overestimated if socioeconomic status is not accounted for. This may explain our results for elderly melanoma patients with stage I disease, whereby the observed survival was better than the expected survival calculated from the general population (relative survival exceeding 100%). However, the relative risk estimate comparing age groups should not be affected, given that the comparability between the cancer group and the external group does not differ across age groups. In a previous Swedish melanoma study, cause-specific survival was similar to relative survival across all age groups [Citation10].
Conclusions
In light of an increasing melanoma burden, it is important to monitor management and survival not the least among young patients. Taken together, our findings show that young adult melanoma patients present with favorable clinical characteristics and receive surgical treatment with short waiting times and in accordance with guidelines. Young adult patients had a better prognosis than older patients, but differences in survival varied by stage of disease at diagnosis and were most prominent in stage II disease. The observed age-related differences in clinical characteristics, management and survival call for an improved understanding of not only disease etiology but also factors driving management decisions. A better understanding of these differences may help improve care and prognosis for melanoma patients of all ages.
Acknowledgements
We thank the Uppsala/Örebro region melanoma group for their continuous work of collecting clinical data.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by the Karolinska Institutet Research Funds and the Swedish Cancer Society (Grant 2012/804).
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v2.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; 2010. [cited 2012 Nov 11]. Available from: http://globocan.iarc.fr.
- Cancerstatistik: The National Board of Health and Welfare (Socialstyrelsen). [cited 2013 May 29]. Available from: www.socialstyrelsen.se/statistik/statistikdatabas.
- Engholm G, Ferlay J, Christensen N, Johannesen TB, Klint Å, Køtlum J, et al. NORDCAN: Cancer incidence, mortality, prevalence and survival in the Nordic countries, Version 5.2: Association of the Nordic Cancer Registries. Danish Cancer Society; 2012. [cited 2013 Jan 25]. Available from: http://www.ancr.nu.
- Aben KK, van Gaal C, van Gils NA, van der Graaf WT, Zielhuis GA. Cancer in adolescents and young adults (15–29 years): A population-based study in the Netherlands 1989–2009. Acta Oncol 2012;51:922–33.
- Purdue MP, Freeman LE, Anderson WF, Tucker MA. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Dermatol 2008;128:2905–8.
- Reed KB, Brewer JD, Lohse CM, Bringe KE, Pruitt CN, Gibson LE. Increasing incidence of melanoma among young adults: An epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc 2012;87:328–34.
- Bleyer A, Barr R. Cancer in young adults 20 to 39 years of age: Overview. Semin Oncol 2009;36:194–206.
- Chagpar RB, Ross MI, Reintgen DS, Edwards MJ, Scoggins CR, Martin RC, et al. Factors associated with improved survival among young adult melanoma patients despite a greater incidence of sentinel lymph node metastasis. J Surg Res 2007;143:164–8.
- Weir HK, Marrett LD, Cokkinides V, Barnholtz-Sloan J, Patel P, Tai E, et al. Melanoma in adolescents and young adults (ages 15–39 years): United States, 1999–2006. J Am Acad Dermatol 2011;65(5 Suppl 1):S38–49.
- Lindholm C, Andersson R, Dufmats M, Hansson J, Ingvar C, Moller T, et al. Invasive cutaneous malignant melanoma in Sweden, 1990–1999. A prospective, population-based study of survival and prognostic factors. Cancer 2004;101:2067–78.
- Lasithiotakis KG, Leiter U, Gorkievicz R, Eigentler T, Breuninger H, Metzler G, et al. The incidence and mortality of cutaneous melanoma in Southern Germany: Trends by anatomic site and pathologic characteristics, 1976 to 2003. Cancer 2006;107:1331–9.
- Tsai S, Balch C, Lange J. Epidemiology and treatment of melanoma in elderly patients. Nat Rev Clin Oncol 2010; 7:148–52.
- Regional rapport 1997-2008: Quality Register of Cutaneous Malignant Melanoma, Regional Cancer Centre in the Uppsala/ rebro region. [cited 2013 April 10]. Available from: http://www.cancercentrum.se/Global/RCCUppsalaOrebro/Vå rdprocesser/malignt_melanom/rapporter/reg_rapport_ 2008.pdf.
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001;19:3635–48.
- Nationellt vårdprogram Malignt hudmelanom: The Swedish Melanoma Study Group; 2007. [cited 2013 Apr 10]. Available from: http://www.karolinska.se/upload/Onkologiskt %20centrum/NationellaVardprogram/Nat_vp_malignt_hudmelanom_2007.pdf.
- Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med 2004;23:51–64.
- Hakulinen T. Cancer survival corrected for heterogeneity in patient withdrawal. Biometrics 1982;38:933–42.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res 2010;23:57–63.
- Siskind V, Whiteman DC, Aitken JF, Martin NG, Green AC. An analysis of risk factors for cutaneous melanoma by anatomical site (Australia). Cancer Causes Control 2005;16:193–9.
- Grange F, Vitry F, Granel-Brocard F, Lipsker D, Aubin F, Hedelin G, et al. Variations in management of stage I to stage III cutaneous melanoma: A population-based study of clinical practices in France. Arch Dermatol 2008;144: 629–36.
- Livingstone E, Windemuth-Kieselbach C, Eigentler TK, Rompel R, Trefzer U, Nashan D, et al. A first prospective population-based analysis investigating the actual practice of melanoma diagnosis, treatment and follow-up. Eur J Cancer 2011;47:1977–89.
- Carpenter S, Pockaj B, Dueck A, Gray R, Kurtz D, Sekulic A, et al. Factors influencing time between biopsy and definitive surgery for malignant melanoma: Do they impact clinical outcome?. Am J Surg 2008;196:834–42; discussion 42–3.
- McKenna DB, Lee RJ, Prescott RJ, Doherty VR. The time from diagnostic excision biopsy to wide local excision for primary cutaneous malignant melanoma may not affect patient survival. Br J Dermatol 2002;147:48–54.
- Kemeny MM, Busch E, Stewart AK, Menck HR. Superior survival of young women with malignant melanoma. Am J Surg 1998;175:437–44; discussion 44–5.
- Lasithiotakis KG, Petrakis IE, Garbe C. Cutaneous melanoma in the elderly: Epidemiology, prognosis and treatment. Melanoma Res 2010;20:163–70.
- Haniff J, de Vries E, Claassen AT, Looman CW, van Berlo C, Coebergh JW. Non-compliance with the re-excision guidelines for cutaneous melanoma in The Netherlands does not influence survival. Eur J Surg Oncol 2006;32:85–9.
- Bilimoria KY, Balch CM, Wayne JD, Chang DC, Palis BE, Dy SM, et al. Health care system and socioeconomic factors associated with variance in use of sentinel lymph node biopsy for melanoma in the United States. J Clin Oncol 2009; 27:1857–63.
- Sarfati D, Blakely T, Pearce N. Measuring cancer survival in populations: Relative survival vs cancer-specific survival. Int J Epidemiol 2010;39:598–610.
- Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: Concepts, methodologies, and guidelines. Annu Rev Public Health 1997;18:341–78.
- MacKie RM, Hauschild A, Eggermont AM. Epidemiology of invasive cutaneous melanoma. Ann Oncol 2009; 20(Suppl 6):vi1–7.

