Abstract
Background. The purpose of this study is to review late toxicity following craniospinal radiation for early-stage medulloblastoma.
Material and methods. Between 1963 and 2008, 53 children with stage M0 (n = 50) or M1 (n = 3) medulloblastoma were treated at our institution. The median age at diagnosis was 7.1 years (range 1.2–18.5). The median craniospinal irradiation (CSI) dose was 28.8 Gy (range 21.8–38.4). The median total dose, including boost, was 54 Gy (range 42.4–64.8 Gy). Since 1963, the CSI dose has been incrementally lowered and the high-risk boost volume reduced. Twenty-one patients (40%) received chemotherapy in their initial management, including 12 who received concurrent chemotherapy. Late sequelae were evaluated by analyzing medical records and conducting phone interviews with surviving patients and/or care-takers. Complications were graded using the NCI Common Terminology Criteria for Adverse Events, version 4.0.
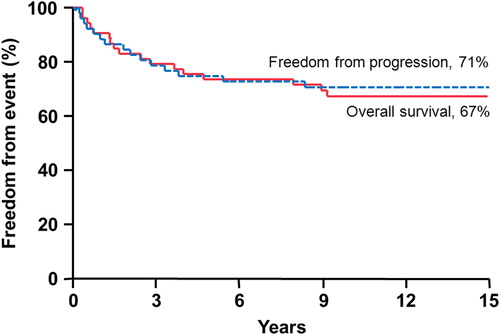
Results. The median follow-up for all patients was 15.4 years (range 0.4–44.4) and for living patients it was 24 years (range 5.6–44.4). The overall survival, cause-specific survival, and progression-free survival rates at 10 years were 67%, 67%, and 71%, respectively. Sixteen patients (41% of patients who survived five years or more) developed grade 3 + toxicity; 15 of these 16 patients received a CSI dose > 23.4 Gy. The most common grade 3 + toxicities for long-term survivors are hearing impairment requiring intervention (20.5%) and cognitive impairment (18%) prohibiting independent living. Four patients developed secondary (non-skin) malignancies, including three meningiomas, one rhabdomyosarcoma, and one glioblastoma multiforme. Three patients (5.6%) died from treatment complications, including radionecrosis, severe cerebral edema, and fatal secondary malignancy.
Conclusion. Ongoing institutional and cooperative group efforts to minimize radiation exposure are justified given the high rate of serious toxicity observed in our long-term survivors. Follow-up through long-term multidisciplinary clinics is important and warranted for all patients exposed to radiotherapy in childhood.
Medulloblastoma is the most common form of malignant pediatric brain tumor, representing approximately 15–30% of all childhood central nervous system (CNS) tumors [Citation1]. About 400–450 new cases of medulloblastoma are diagnosed in the USA each year [Citation2] in patients under 21 years of age.
The introduction of postoperative radiotherapy (RT) in the 1950s has resulted in a significant improvement in overall survival (OS) in patients diagnosed with medulloblastoma [Citation1]. Surgery alone was largely unsuccessful due to frequent local recurrence and leptomeningeal spread along the craniospinal axis [Citation1]. Current treatment strategies include maximal safe resection of the primary lesion followed by craniospinal irradiation (CSI) with concurrent and adjuvant chemotherapy.
Although the standard integration of adjuvant RT in the treatment paradigm for medulloblastoma has markedly increased OS, complications associated with neuro-axis irradiation are a significant source of treatment-associated morbidity and mortality. Multiple studies have demonstrated a dose-dependent decline in neurocognitive function after whole-brain RT, with more serious deficits seen in younger patients. Other late serious complications attributable at least in part to RT include abnormalities in growth and development, endocrine dysfunction, hearing deficits, visual disturbances, cardiovascular toxicity, gonadal dysfunction, and radiation-induced secondary malignancies.
In light of the risk of late effects following adjuvant CSI, the standard of care for medulloblastoma has continued to evolve since the 1950s, with gradual shifts towards dose reduction and changes aimed at minimizing treatment-related toxicity while maintaining comparable OS. Thus, long-term follow-up for patients with medulloblastoma is critical not only in evaluating treatment efficacy, but also in evaluating possible means of improving quality of life and avoiding serious complications.
The aim of this study is to review survival outcomes for a single-institution cohort of pediatric patients and evaluate the range of long-term sequelae after CSI for medulloblastoma.
Material and methods
Under institutional review board approval, we conducted a review of pediatric patients 18 years of age or younger with histologically-proven primary medulloblastoma who received adjuvant photon RT with curative intent at our institution. Patients who were treated with palliative intent or presented with overt metastatic disease were excluded from this study. The minimal potential follow-up was five years. We identified 53 patients treated between October 1963 and December 2008 who met the inclusion criteria for analysis. Patient characteristics are summarized in . There were 15 (28%) females and 38 (72%) males. The median patient age at diagnosis was 7.1 years (range 1.2–18.5 years). Forty-eight patients (91%) were white and five (9%) were black.
Table I. Patient, tumor, and treatment characteristics.
Disease stage according to the Chang guidelines for M staging of medulloblastoma were as follows: M0 stage, 50 patients; M1 stage, three patients. At the time of diagnosis, 45 patients (85%) had confirmed hydrocephalus. Four patients had other notable medical conditions, including (one of each) coarctation of the aorta, Gorlin's syndrome, Hashimoto's thyroiditis, and a patent ductus arteriosus at the time of diagnosis. Gorlin's syndrome has a well-known association with medulloblastoma due to an inherited protein patched homolog (PTCH) gene mutation.
Treatment
All patients in this study underwent primary surgery and postoperative RT. Assessment of the extent of resection was based on available operative notes, postoperative magnetic resonance imaging (MRI) reports, and clinic notes from treating physicians. Subtotal resection (STR) was defined as more than 1.5 cm2 of residual disease, while near total resection (NTR) was defined as residual tumor measuring less than 1.5 cm2. Gross total resection (GTR) was defined as complete macroscopic tumor resection. When available, the surgeon's assessment of the extent of resection in the operative note was confirmed by the findings on postoperative MRI. After initial surgery, 19 patients (36%) had a GTR, nine patients (16%) had an NTR, and 25 patients had an STR (47%). Of note, one patient who had an STR underwent a second-look surgery one week after initial surgery to achieve a GTR.
RT dosing strategies varied considerably among treatment eras. In the early era, radiation was delivered daily to moderately high CSI doses in the absence of chemotherapy. Radiation dose and intensity was then modified though hyperfractionation. When concurrent chemoradiation was introduced, daily fractionation with lower total radiation doses were the norm. Across all patients in this study, the median total RT dose was 54 Gy (range 42.4–64.8 Gy), with a median initial CSI dose of 28.8 Gy (range 21.8–38.4 Gy) and a median posterior fossa or tumor bed boost of 25 Gy (range 14.1–38.4 Gy). The median time interval between surgery and the start of RT was 20 days (range 5–160 days). The median RT treatment duration was 45 days (range 30–65 days). The median time interval from surgery to the completion of RT was 69 days (range 42–217 days). The disparity in time intervals between surgery and the start or completion of RT depended on whether the patient received pre-RT chemotherapy as well as the presence of postoperative sequelae requiring convalescence before initiating RT. The patient with the longest time interval from surgery to the end of RT was three years old at initial presentation and received neoadjuvant MOPP (mustargen, oncovin, procarbazine, and prednisone), cisplatin, vincristine (VCR), and etoposide prior to receiving adjuvant RT. Most patients (81%) received once-daily RT, while seven patients had twice-daily treatment and three patients had combined once- and twice-daily treatment. All patients were treated with cobalt-60 (n = 4) or linear accelerator-based photon therapy.
All patients in the series had a histologically- confirmed diagnosis of medulloblastoma; historically the pathology reports did not consistently include a more specific subclassification. Six patients had confirmed desmoplastic histology, one had anaplastic histology, and 46 had a classic or unknown subtype. Chemotherapy regimens also varied considerably among treatment eras. In total, 21 patients (39.6%) received chemotherapy in addition to surgery and RT; however, only two patients treated before 1990 received chemotherapy. The most common chemotherapy regimens were as follows: concurrent VCR with adjuvant VCR, lomustine, and cisplatin ± adjuvant etoposide/cytoxan (n = 6); or neo-adjuvant etoposide and cisplatin ± adjuvant VCR/cytoxan (n = 5); or concurrent VCR with adjuvant VCR, cytoxan, cisplatin ± adjuvant etoposide/carboplatin (n = 2). Less than 5% of patients received heterogeneous chemotherapy regimens that involved MOPP, adriamycin, and lomustine. Six patients received neoadjuvant chemotherapy, 12 patients received concurrent chemotherapy, and 18 patients received adjuvant chemotherapy (because many patients received chemotherapy spanning treatment phases, the number of regimens listed exceeds the total number of patients who received chemotherapy).
Follow-up
After informed consent to participate in the study was obtained, patients were interviewed using an informal script. Patients or caretakers were questioned about various quality-of-life measures, including their learning abilities, ability to maintain employment, or ability to live and care for themselves independently. The telephone interview included an evaluation of the presence of common sequelae in children treated with RT for medulloblastoma, including but not limited to the following: hearing deficits, cognitive impairments, visual disturbances, endocrine dysfunction, cerebrovascular complications, seizures, and secondary malignancy development. Inquiries were made regarding follow-up with physicians and specialists for areas such as vision, hearing, neurocognition, neuroendocrine, cardiac, and reproductive issues.
Serious complications were defined as any complication classified as grade 3, 4, or 5 as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. In general grade 3 complications are serious, medically significant, but not life threatening, grade 4 complications require urgent intervention and may be life-threatening, and grade 5 toxicities lead to death.
SAS and JMP software were utilized for all statistical computations (SAS Institute, Cary, NC, USA). The Kaplan-Meier product limit method provided estimates of OS, cause-specific survival (CSS), progression free survival (PFS).
Results
Outcomes
The median follow-up from diagnosis was 15.4 years (range 0.4–44.4 years), with a median follow-up of surviving patients of 24.0 years (range 5.6–44.4 years). There were 34 surviving patients at the time of the analysis. Two patients were lost to follow-up at 13.3 and 36.3 years; three patients were confirmed to be alive with no evidence of disease (NED) via our institution's tumor registry and/or a certified letter; and we were able to contact 29 patients by phone for follow-up. For the entire group, the actuarial five- and 10-year PFS rates were 75% and 71%, respectively. CSS and OS rates were the same at five and 10 years: 74% and 67%, respectively ().
Acute toxicity
Acute toxicities recorded during the course of RT most frequently included nausea and vomiting in 34 patients (64%), which was usually mild or controlled with medication. Two patients had serious nausea and vomiting accompanied by anorexia, refractory to antiemetics and requiring nasogastric enteral support. Other acute toxicities reported during treatment were uncommon and mild, including dry mouth, headache, mucositis, and back pain.
Late toxicity
Analysis of late treatment toxicities includes only data from the 39 patients (73.6%) surviving over five years after initial surgery. Grade 1 toxicity was not recorded. Late treatment toxicities are summarized in .
Table II. Number of registered complications by grade: N = 39 patients with > 5 years of follow-up.
The most commonly reported sequela was growth suppression in 24 patients (61.5%). There was insufficient information in the medical records to formally grade the growth suppression according to CTCAE guidelines. These patients had medical records indicating a reduction in growth velocity or decreased percentile compared to a prior growth curve, or they claimed notable short stature compared to siblings and expected height. A total of 10 long-term survivors reported having received growth hormone supplementation (25.6%).
Cognitive impairment was reported by 49% (N = 19) of long-term survivors. Problems included difficulty learning, poor concentration, poor memory, and antisocial behavior. Hearing loss was reported in 39% (N = 15) of patients. Hypothyroidism requiring hormone replacement was reported in 21% (N = 8) of long-term surviving patients; all affected patients were euthyroid on levothyroxine therapy. Other late effects included adrenocorticotropic hormone deficiency, osteoporosis, encephalopathy, low testosterone, cataracts, mineralizing microangiopathy, ataxia, facial nerve palsy, muscle weakness, fertility issues, and radiation-induced serous otitis media.
Serious treatment toxicity
Sixteen patients (41% of long-term survivors) had serious complications after treatment, which could be attributed at least in part to RT. The CSI dose delivered was greater than 23.4 Gy in 94% (15 of 16) of patients with serious late sequelae. More than half (56%) of the 16 patients with serious complications had more than one grade 3 + complication. Seven of the long-term survivors with serious late sequelae also received chemotherapy during initial treatment, and all of their regimens included cisplatin. shows all grade 5 complications (deaths related to treatment).
Table III. Fatal complications and patient and treatment characteristics.
The most common serious late complication was grade 3 hearing loss requiring medical intervention, which occurred in 20.5% (8/39) of long-term survivors. Four patients (50%) with grade 3 hearing loss were treated with chemotherapy, including cisplatin, and now require hearing aids. One of these patients developed unilateral hearing loss attributed to radiation-induced serous otitis media and sensorineural hearing loss. Another patient had unilateral hearing loss due to osteoradionecrosis of the temporal bone.
Eighteen percent (7 of 39) of the long-term survivors reported grade 3 cognitive impairment, indicating they were incapable of independent living. Six were age 7 or younger at the time of RT, while one child was 11 years old at the time of RT. Commonly reported indications for adult children living with their parents included emotional liability and behavioral dysfunction as well as extreme short-term memory impairment and inability to maintain employment. Of these seven patients, three individuals have serious cognitive impairment and behavior requiring placement in a long-term care facility and/or non-residential healthcare facility.
Various non-skin secondary malignancies developed in four patients (10% of long-term survivors). Two patients developed meningiomas requiring surgical excision at 8.7 and 11.9 years after RT. One patient developed a frontal meningioma 13.7 years after treatment that has not required surgical intervention. One patient developed a rhabdomyosarcoma in the right nasal cavity 6.4 years after treatment for medulloblastoma. This tumor was successfully treated with RT and chemotherapy. One patient developed a glioblastoma multiforme (GBM) 14.4 years after RT. The GBM was treated with RT and cetuximab. This patient died within a year of diagnosis of the GBM.
Three patients developed radionecrosis. The first patient developed osteoradionecrosis of the temporal bone 1.8 years after RT associated with injury of cranial nerve VIII. Despite hyperbaric oxygen treatments and surgical intervention, the patient ultimately lost hearing in the ipsilateral ear. A second patient developed radionecrosis of the left occipital lobe 2.8 years after treatment and required surgery, which was complicated by the development of right homonymous hemianopsia. A third patient developed radionecrosis involving the cerebellum 1.5 years after completing RT and ultimately succumbed to cerebellar degeneration.
Two patients in our series experienced serious (grade 3+) cerebrovascular long-term sequelae. One patient suffered a right medullary infarct at the age of 30 years old, 23.7 years after CSI. This patient was unable to maintain employment and required assistance from his parents at the time of this analysis. A second patient had multiple transient ischemic attacks at age 29 years old, 20.5 years after treatment, and then was subsequently diagnosed with a fusiform basilar artery aneurysm at the age of 43 years old, 34.5 years following treatment. No patients developed a grade 3 or higher cardiac toxicity. Only one patient developed grade 2 cardiac toxicity, asymptomatic myocardial infarction detected on electrocardiogram before he reached 45 years old. One patient developed serious ataxia including progressive loss of coordination and dysarthria 20 years after RT, attributed to treatment and affecting instrumental activities of daily living and employment. Seizures refractory to oral medication have been reported by one individual, with seizures beginning 19.4 years after treatment for medulloblastoma. This patient was unable to maintain employment because of the frequency and severity of seizures.
outlines these various serious complications stratified by time to occurrence. Over the last five decades, the CSI dose and posterior fossa boost target volume have been incrementally reduced in M0 patients. Despite the intent of decreased treatment volume and decreased total CSI dose, there was no clear trend showing decreased toxicity among patients with more modern treatment plans.
Table IV. Time from initial surgery to occurrence of serious toxicity.
Despite many patients reporting grade 2 or higher late toxicities from their medulloblastoma treatment, most patients rated themselves as healthy during phone interviews. Although we did not objectively evaluate quality of life using a validated questionnaire, the majority of patients contacted were able to complete high school and most patients are able to maintain professional level employment.
Discussion
Late sequelae
As survival rates for medulloblastoma continue to improve with time, observation and long-term follow-up of late sequelae are imperative. We have become especially familiar with long-term side effects in children treated with CSI. Few studies have long-term follow-up over 10 years [Citation3]. Our study has a 15-year median follow-up for all patients and 24-year median follow-up of surviving patients, allowing for thorough examination of late toxicity.
Furthermore, many studies focus on one or two long-term complications, such as hearing loss [Citation4] or endocrine and cardiac deficiency [Citation5]. This study comprehensively examined multiple organ systems and potential associated late complications of therapy.
Reductions in CSI dose and boost-tumor volume over the last five decades have not compromised disease control as evidenced by our survival rates, which are similar to those in the published literature [Citation3]. With high five- and 10-year survival rates in M0/M1 children, studies are shifting focus to minimizing long-term sequelae related to high-dose radiation.
Neurocognitive impairment
The detrimental effects on quality of life for patients and caretakers due to cognitive impairment warrant studies into dose reduction and minimizing toxicity. In our cohort, nearly 20% of patients surviving their disease for over five years experienced serious cognitive impairment.
Abundant data correlate medulloblastoma treatment with a decline in neurocognitive function [Citation1,Citation6,Citation7]. Late neurocognitive dysfunction is attributed to whole brain irradiation, and it is expected that deficits in decision making and memory are related to irradiation of the frontal and temporal regions [Citation6]. Armstrong et al. assessed region-specific radiation dose and self-reported neurocognitive function of 818 patients, and found that patients receiving a dose of ≥ 30 Gy to the temporal region were at an increased risk of memory impairment.
There is also a strong correlation of young age at the time of RT with more serious late cognitive impairments [Citation7]. The immature brain is more susceptible to neurocognitive impairments induced by radiation. A prospective, longitudinal study of 111 patients by Mulhern et al. found young age at diagnosis (< 7 years old) to be the most prominent risk factor for developing neurocognitive impairment for medulloblastoma patients.
There is literature supporting the notion that a child who presents with hydrocephalus might experience poorer neurological outcomes [Citation8]. While we were able to confirm that many of the children in our cohort did experience hydrocephalus, our raw data is inconclusive: three of eight (37%) children without hydrocephalus had grade 3 + toxicity and four of 45 (9%) children with hydrocephalus experienced similar morbidity. These values failed to reach statistical significance (p = 0.2878). Hydrocephalus likewise was not specifically associated with neurocognitive impairment.
Although age at treatment with RT increases the risk of neurocognitive impairment, this risk is present for all patients treated with CSI. A longitudinal study on 20 survivors of medulloblastoma also showed a continued decline in working memory as time from diagnosis progressed. These findings suggest that the cognitive impairments due to RT may lead to a continuous decline in neurocognitive function for even adult survivors of medulloblastoma.
The median age at the time of RT for the seven patients in our study who developed grade 3 neurocognitive impairment was 5.4 years old which was not significantly younger than the median age of children without cognitive impairments (p = 0.0877). Our data were unable to show a significant difference, but suggests a trend that is consistent with findings in other studies that younger children will develop neurocognitive impairment following RT more frequently than older children. The sample size of our population with severe cognitive impairment was small (n = 7), which may be why it fails to differentiate between median age between the groups.
Ototoxicity
Polkinghorn et al. retrospectively studied radiation ototoxicity in 33 children treated for medulloblastoma between 1999 and 2007. The study had a median follow-up of 19 months, and 6% of their patients developed serious grade 3 hearing loss warranting intervention [Citation4]. Our study has longer median follow-up, with 20.5% (eight of 39 patients) of those patients who survived over five years developing grade 3 hearing deficits. Of note, this cohort includes patients treated with older RT techniques and many of our patients did not receive ototoxic chemotherapy.
Endocrine defects
RT can damage the pituitary gland, thyroid, and hypothalamus in patients treated for medulloblastoma, resulting in endocrine defects [Citation1,Citation5]. Growth hormone deficiency is the most common endocrinopathy in patients treated for medulloblastoma, resulting in short stature as a common sequela [Citation1]. In long-term survivors of our study, subjective short stature was reported in 24 of 39 patients (61.5%). Only 10 patients reporting short stature also reported growth hormone replacement therapy, which may indicate the subjective nature of these data or suboptimal intervention. Direct radiation to the vertebral column can also contribute to growth suppression [Citation1]. The relationship between age at the time of RT and subsequent changes in growth hormone level is unclear [Citation1], but it does appear that age at the time of RT influences vertebral body growth rate, likely due to direct effects of radiation on bone structure.
Primary or central hypothyroidism may develop after CSI [Citation9]. The exit dose of spinal axis RT may damage the thyroid directly, or RT to the hypothalamic-pituitary axis may result in central hypothyroidism. Paulino et al. evaluated thyroid function in 32 patients treated with CSI for medulloblastoma and found that after a median follow-up of 41 months, 56% of patients developed hypothyroidism. The study by Paulino has a higher rate of hypothyroidism than our own study, but this may be owing to the fact that those authors did not differentiate between grade 1 (observed deficiency on blood labs only) and grade 2 hypothyroidism (low lab values warranting thyroid replacement therapy). In that study, younger age at treatment and chemotherapy correlated with a higher incidence of hypothyroidism.
Radionecrosis
Our study includes three cases of reported necrosis: one case of osteoradionecrosis and two cases of brain necrosis. All cases occurred between one and three years after treatment. Murphy et al. retrospectively reviewed the medical records of 236 children treated between 1996 and 2009 for medulloblastoma or other CNS embryonal tumors treated with postoperative RT and adjuvant chemotherapy including cisplatin, vincristine, and cyclophosphamide. The study had a median follow-up of 52 months, with eight cases (3.4%) of reported necrosis, including one death. Murphy et al. concluded that the volume of infratentorial brain receiving RT doses of > 50 Gy, > 52 Gy, and > 54 Gy is predictive of necrosis [Citation10].
Secondary malignancies
Secondary malignancies are among the most serious long-term sequelae in children treated for childhood brain tumors. Long-term follow-up beyond 10 years is vital to accurately characterize the incidence rate [Citation1]. The relative risk for secondary malignancies in medulloblastoma has been calculated between 4.59 and 20 [Citation11–13], and does not plateau or decline after 10 years [Citation12]. Children treated for medulloblastoma may experience a variety of secondary malignancies including leukemias and solid tumors of the urinary or digestive tracts, thyroid, and CNS [Citation11].
Non-skin malignancies in this cohort include five secondary malignancies in the RT field including one nasal cavity rhabdomyosarcoma, one GBM, and three meningiomas, diagnosed between 6.4 and 14 years after initial treatment, respectively. A Surveillance, Epidemiology, and End Results (SEER) Program study done by Strodtbeck et al. on secondary malignancy after RT for medulloblastoma or primitive neuroectodermal tumors demonstrated that secondary brain malignancies can be diagnosed as early as one year to over 10 years after initial treatment [Citation11]. Case reports have documented second tumors arising up to 35 years after initial treatment for medulloblastoma [Citation14]. These findings highlight the importance of lifelong follow-up for patients treated with CSI for childhood brain cancers.
Cerebrovascular toxicity
The most common radiation-induced vascular alterations are thrombosis and occlusive cerebrovascular changes, and less often radiation results in aneurysm formation. It is hypothesized that occlusive vasculopathy and aneurysms form as a result of radiation-induced endothelial damage. A study of 1817 childhood brain tumor survivors demonstrated that cranial radiation doses of > 30 Gy lead to an increased risk of stroke [Citation15].
In our study, two patients experienced cerebrovascular toxicity. One patient developed a right medullary infarct after whole-brain irradiation to 30 Gy as management for his medulloblastoma. The second patient developed a fusiform basilar aneurysm and multiple transient ischemic attacks. This second patient was treated with 40 Gy to the whole brain. The high whole-brain doses in these patients with cerebrovascular toxicity are consistent with findings from Bowers et al. regarding dose-dependent relationship of late cerebrovascular events.
Benson et al. report on three cases of RT-induced aneurysms following treatment for pediatric medulloblastoma, all which ended in rupture and death nine to 19 years after RT treatment [Citation16]. Case reports like this demonstrate that cerebrovascular changes after CSI are a rare yet potentially devastating late effect.
Cardiovascular disease
Based on the frequency of cardiac events in Hodgkin lymphoma survivors, we hypothesized that patients might experience cardiac toxicity from CSI treatment because of the radiation exit dose. In this study, only one patient developed cardiac toxicity, with an asymptomatic grade 2 myocardial infarction detected on electrocardiogram.
In a large study by Gurney et al. of endocrine and cardiovascular late events for survivors of childhood brain tumors, there were two cases of arrhythmias reported and 35 cases of angina-like symptoms in a cohort of 343 patients treated with RT for medulloblastoma or primitive neuroectodermal tumors [Citation5]. There is little published data on cardiac toxicity after treatment for medulloblastoma.
Mulrooney et al. retrospectively evaluated a large cohort of survivors of pediatric cancer compared with sibling controls, and found that cardiac irradiation ≤ 15 Gy did not significantly increase the hazard ratio of heart failure and myocardial infarction in patients treated with radiation compared to those who did not receive radiation. Another study on cardiovascular death in patients treated with RT for childhood cancers calculated a risk ratio of 12.5 for cardiovascular death due to RT (95% CI 1.4–116) for doses ranging from 5 to 14.9 Gy [Citation17]. While these data demonstrate that cardiovascular toxicity can develop in children treated with RT, they do not specifically address the risks of CSI and late cardiac sequelae.
Limitations
Limitations of the current study are similar for all retrospective studies involving rare tumors. Namely, the findings from our study are hypothesis-generating and cannot prove a causal relationship. The relatively small number of patients and events prohibit a formal statistical analysis. To provide the long-term follow-up data, the study includes patients treated over five decades; therefore, staging and treatment heterogeneity across treatment eras is inevitable. For example, most of our patients (58%) were treated before 1991 when chemotherapy was not the standard of care. As the standard of care has evolved over the decades so too has the late-effect profile for medulloblastoma survivors. Increasing use of chemotherapy regimens and lower radiation doses yield higher rates of late toxicities, such as acute leukemias, myelosuppression, and chronic peripheral neuropathy. Yet despite this study's limitations, it provides unique insight into sequelae caused by craniospinal RT in medulloblastoma patients decades following treatment.
Future developments
The study limitations outlined above illustrate that future interventions intended to reduce radiation toxicity in patients with medulloblastoma should ideally be guided by prospective studies. To this end, our institution is currently participating in national protocols, such as Children's Oncology Group ACNS0331, a randomized study for patients with standard-risk medulloblastoma that evaluates lower-dose CSI and a smaller boost target, and SJYC07, a risk-stratified study under which infants with standard-risk medulloblastoma receive high-dose methotrexate-based chemotherapy followed by focal radiation alone. Newer studies, such as SJMB12, offer a risk-adapted approach to craniospinal RT based on molecular subgroup. We also offer all pediatric patients with medulloblastoma enrollment on a prospective institutional study designed to systematically assess the reduced acute and late toxicity in children treated with proton therapy. Meticulously monitoring toxicities is especially important, as proton therapy may reduce the endocrinopathy, vasculopathy, neurotoxicity, ototoxicity, and second CNS malignancies observed in our historic cohort [Citation18–23]. Various authors also cite decreased cardiac and pulmonary toxicities, and a reduced risk of non-CNS second malignancies, as indications for proton therapy in patients requiring CSI [Citation18,Citation24–26]. While these benefits are evident from a dosimetric standpoint, our data did not show an increased incidence for these complications, and thus longer follow-up might be required to evaluate the absolute potential benefit of proton therapy in these areas.
Conclusion
As treatment for medulloblastoma continues to improve OS and outcomes, long-term evaluation of patients is critical. The numerous secondary effects of RT may develop over decades and these patients will require lifelong specialized medical care. Further prospective studies investigating how to minimize late sequelae will be important in improving the quality of life of long-term survivors.
While there were significant reductions in CSI dose and boost target volume over the length of this study, we have not observed a definitive correlative decrease in serious toxicity in the modern era. Ongoing institutional and cooperative group efforts to minimize radiation exposure are justified given the high rate of serious toxicity observed in these long-term survivors. For survivors of pediatric medulloblastoma, the value of long-term follow-up clinics and continuity of multidisciplinary medical care is indisputable.
Acknowledgments
We would like to thank Jessica Kirwan and the editorial staff of the Department of Radiation Oncology, University of Florida for editing and preparing this manuscript for publication.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Fossati P, Ricardi U, Orecchia R. Pediatric medulloblastoma: Toxicity of current treatment and potential role of protontherapy. Cancer Treat Rev 2009;35:79–96.
- Gajjar A, Packer RJ, Foreman NK, Cohen K, Haas-Kogan D, Merchant TE, et al. Children’s Oncology Group’s 2013 blueprint for research: Central nervous system tumors. Pediatr Blood Cancer 2013;60:1022–6.
- Brasme JF, Grill J, Doz F, Lacour B, Valteau-Couanet D, Gaillard S, et al. Long time to diagnosis of medulloblastoma in children is not associated with decreased survival or with worse neurological outcome. PloS One 2012;7:e33415.
- Polkinghorn WR, Dunkel IJ, Souweidane MM, Khakoo Y, Lyden DC, Gilheeney SW, et al. Disease control and ototoxicity using intensity-modulated radiation therapy tumor-bed boost for medulloblastoma. Int J Radiat Oncol Biol Phys 2011;81:e15–20.
- Punyko JA, Mertens AC, Gurney JG, Yasui Y, Donaldson SS, Rodeberg DA, et al. Long-term medical effects of childhood and adolescent rhabdomyosarcoma: A report from the childhood cancer survivor study. Pediatr Blood Cancer 2005;44:643–53.
- Armstrong GT, Jain N, Liu W, Merchant TE, Stovall M, Srivastava DK, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neurooncology 2010;12:1173–86.
- Mulhern RK, Palmer SL, Merchant TE, Wallace D, Kocak M, Brouwers P, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol 2005;23:5511–9.
- Duff D. Ageism, elitism, and anti-intellectualism in nursing. Axone 2005;26:4–5.
- Paulino AC. Hypothyroidism in children with medulloblastoma: A comparison of 3600 and 2340 cGy craniospinal radiotherapy. Int J Radiat Oncol Biol Phys 2002;53:543–7.
- Murphy ES, Merchant TE, Wu S, Xiong X, Lukose R, Wright KD, et al. Necrosis after craniospinal irradiation: Results from a prospective series of children with central nervous system embryonal tumors. Int J Radiat Oncol Biol Phys 2012;83:e655–60.
- Strodtbeck K, Sloan A, Rogers L, Fisher PG, Stearns D, Campbell L, et al. Risk of subsequent cancer following a primary CNS tumor. J Neuro-oncol 2013;112:285–95.
- Stavrou T, Bromley CM, Nicholson HS, Byrne J, Packer RJ, Goldstein AM, et al. Prognostic factors and secondary malignancies in childhood medulloblastoma. J Pediatr Hematol Oncol 2001;23:431–6.
- Goldstein AM, Yuen J, Tucker MA. Second cancers after medulloblastoma: Population-based results from the United States and Sweden. Cancer Cause Control 1997;8:865–71.
- Hamasaki K, Nakamura H, Ueda Y, Makino K, Kuratsu J. Radiation-induced glioblastoma occurring 35 years after radiation therapy for medulloblastoma: Case report. Brain Tumor Pathol 2010;27:39–43.
- Bowers DC, Liu Y, Leisenring W, McNeil E, Stovall M, Gurney JG, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: A report from the Childhood Cancer Survivor Study. J Clin Oncol 2006;24:5277–82.
- Benson PJ, Sung JH. Cerebral aneurysms following radiotherapy for medulloblastoma. J Neurosurg 1989;70:545–50.
- Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, Haddy N, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 2010;28:1308–15.
- Zhang R, Howell RM, Giebeler A, Taddei PJ, Mahajan A, Newhauser WD. Comparison of risk of radiogenic second cancer following photon and proton craniospinal irradiation for a pediatric medulloblastoma patient. Phys Med Biol 2013;58:807–23.
- Howell RM, Giebeler A, Koontz-Raisig W, Mahajan A, Etzel CJ, D’Amelio AM, Jr., et al. Comparison of therapeutic dosimetric data from passively scattered proton and photon craniospinal irradiations for medulloblastoma. Radiat Oncol 2012;7:116.
- Moeller BJ, Chintagumpala M, Philip JJ, Grosshans DR, McAleer MF, Woo SY, et al. Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiat Oncol 2011;6:58.
- Kahalley L, Okcu MF, Ris MD, Grosshans D, Paulino A, Chintagumpala M, et al. IQ change within three years of radiation therapy in pediatric brain tumor patients treated with proton beam radiation therapy versus photon radiation therapy. J Clin Oncol 2013;31(Suppl; abstr 10009).
- Yock TI, Yeap BY, Pulsifer MB, Ebb D, MacDonald SM, Marcus KC, et al. Results from a prospective trial of proton radiotherapy for medulloblastoma: Clinical outcomes including hearing and neurocognitive. Int J Radiat Oncol Biol Phys 2011;81:S113.
- Moeller BJ, Chintagumpala M, Philip JJ, Woo SY, Wolff JE, Mahajan A. Proton radiotherapy for pediatric medulloblastoma: Improved early ototoxicity. Int J Radiat Oncol Biol Phys 2010;78:S18.
- Zhang R, Howell RM, Homann K, Giebeler A, Taddei PJ, Mahajan A, et al. Predicted risks of radiogenic cardiac toxicity in two pediatric patients undergoing photon or proton radiotherapy. Radiat Oncol 2013;8:184.
- Brodin NP, Vogelius IR, Maraldo MV, Munck af Rosenschold P, Aznar MC, Kiil-Berthelsen A, et al. Life years lost – comparing potentially fatal late complications after radiotherapy for pediatric medulloblastoma on a common scale. Cancer 2012;118:5432–40.
- Brodin NP, Munck Af Rosenschold P, Aznar MC, Kiil- Berthelsen A, Vogelius IR, Nilsson P, et al. Radiobiological risk estimates of adverse events and secondary cancer for proton and photon radiation therapy of pediatric medulloblastoma. Acta Oncol 2011;50:806–16.