Abstract
Background. Physical activity during chemotherapy has been shown in several studies to reduce fatigue, improve symptoms and impact positively on health-related quality of life (HRQoL). Challenges associated with intervention studies on physical activity during cancer treatment relate to consistent adherence. The primary objective was to study feasibility and adherence of physical activity intervention among patients with cancer during adjuvant chemotherapy treatment. The secondary objective was to investigate the effects of physical activity on health aspects, including HRQoL, symptoms and surrogate markers for cardiovascular disease.
Material and methods. This randomized controlled trial included patients with breast cancer (BRCA) and colorectal cancer (CRC) during adjuvant chemotherapy. The intervention continued for 10 weeks and included daily walks of 10 000 steps and a weekly supervised group walk. Adherence was assessed by a pedometer and the number of participants who reported step counts every week and percentage of participants who achieved the target steps every week.
Results. Adherence average reached 91% during the intervention period; in total 74% completed the exercise intervention. The majority of the participants achieved an average of 83% of the target of 10 000 steps per day for 10 weeks. There was a significant increase in daily physical activity (p = 0.016) in the intervention group. Significant differences were also found for some breast cancer-specific symptoms [swelling, mobility and pain (p = 0.045)]. The study showed a relatively small weight gain an average of 0.9 kg in the intervention group and 1.3 kg in the control group.
Conclusion. Physical activity in the form of walking is feasible during adjuvant chemotherapy treatment despite increasing symptoms. The physical activity increased in the intervention group during the study time and had a positive impact on breast symptoms and the weight gain was lower in comparison to previous studies.
The majority of the research into physical activity during and after treatment for cancer indicates that it has a positive impact on prognosis. Epidemiological data suggest that physical activity reduces the risk of a diagnosis of breast cancer (BRCA) or colorectal cancer (CRC) and several studies of physical activity after diagnosis report less risk of recurrence and improved survival in patients with cancer [Citation1–3]. To date studies have mostly been based on retrospective and observational data and were usually conducted after treatment was completed. There is also a lack of evidence in terms of prospective randomized controlled trials during chemotherapy treatment that evaluates optimal dose, type of exercise, timing and length of exercise to prevent common adverse side effects of cancer therapy [Citation4,Citation5]. Fatigue is generally one of the most reported side effects of chemotherapy; 90–100% of the patients suffer during treatment and it affects patients’ health-related quality of life (HRQoL) negatively [Citation6]. Patients may also have to adjust their daily activities and often become more inactive after a diagnosis of cancer. Fatigue correlates with many other known side effects, such as shortness of breath, nausea, sleep disturbance, pain and anemia [Citation6]. Patients with BRCA commonly report an impaired HRQoL during and after chemotherapy treatment and HRQoL has also been seen as a prognostic factor that correlates to survival and response to treatment [Citation7]. An impaired HRQoL is also reported by patients diagnosed with CRC in some studies [Citation8]. Physical activity as an intervention has been shown to improve both functional and overall HRQoL in populations of patients with BRCA and CRC during and after chemotherapy treatment. HRQoL improvements related to physical activity have been seen in self-confidence, wellbeing and reduced anxiety levels [Citation3,Citation9–11]. Being physically active during chemotherapy treatment can also reduce fatigue, improve fitness and muscle strength, and either decrease or maintain body weight [Citation12].
Obesity not only increases the risk of developing BRCA and CRC [Citation13,Citation14], it also seems to impact on the outcome after diagnosis [Citation2,Citation15]. Weight gain for women treated for BRCA has been found to be associated with BRCA treatment and adjuvant chemotherapy [Citation14,Citation15], the frequency of which has been reported to range from 60% to 84%. It is not uncommon that women gain 5% or more of their starting weight [Citation15], and there is also evidence that patients who gain weight during treatment are more likely to have an unfavorable prognosis [Citation14,Citation15]. Reasons for weight gain during treatment may be caused by the use of steroids, inactivity related to side effects such as fatigue, and the loss of muscle mass leading to reduced energy expenditure and hormonal changes [Citation15]. A diagnosis of diabetes during cancer and cancer treatment can also affect prognosis and survival in both the CRC and BRCA population [Citation16,Citation17]. Adjuvant chemotherapy may contribute to metabolic syndrome. Metabolic syndrome comprises obesity in combination with elevated cholesterol (HDL, LDL and triglycerides), blood pressure and blood glucose levels. Activity level, weight and metabolic balance are closely linked [Citation18] and physical activity is an important part of the treatment of metabolic syndrome [Citation19]. The symptoms from metabolic syndrome are strongly linked to cardiovascular morbidity and mortality in patients with cancer [Citation15]. Steroids are commonly used to alleviate side effects and a known side effect of steroid use is reduced insulin sensitivity [Citation16]. There is evidence that physical activity reduces insulin resistance in both healthy individuals and patients with cancer [Citation20]. In BRCA-specific studies, during adjuvant therapy, physical activity has been shown to improve blood lipid levels [Citation11], cardio- respiratory fitness with reduced systolic resting pressure and heart rate [Citation4].
Adherence to physical activity interventions
Challenges associated with intervention studies on physical activity for patients during cancer treatment relate to consistent adherence [Citation21,Citation22]. Adherence to physical activity is a critical concern because the benefits of physical activity are not always seen immediately. Even for healthy people, adherence to training is cumbersome, and the challenge becomes even more difficult after a diagnosis and during treatment [Citation23]. Intervention studies with physical activities such as walking or aerobic exercise during adjuvant treatment reported a wide range of levels of adherence; between 26% and 93%. Moderate intensity interventions lasting up to 6 weeks and sessions taking place about two or three times per week report higher adherence rates [Citation24]. These results imply that interventions with lower prescribed doses of exercise present greater adherence rates than those with higher doses. Adherence is also linked to a well- designed physical activity intervention that allows easy assessment for self-reporting of activity and includes the ability to monitor and control the physical activity [Citation21,Citation22]. Research to date indicates that physical activity during and after chemotherapy causes no greater risk of adverse events [Citation9,Citation12], but currently there is not enough evidence to determine what form it should take or at what intensity it should be set in order to be optimal during cancer treatment [Citation5].
The primary objective of this study was to investigate the feasibility and adherence of a physical activity intervention among patients with BRCA and CRC during adjuvant chemotherapy treatment. The secondary objective was to investigate the effects of physical activity on health aspects, including quality of life and symptoms, and measure surrogate markers for cardiovascular disease (CVD).
We hypothesized that it was feasible to implement a physical activity intervention for patients during adjuvant chemotherapy treatment and that it would be possible for patients to adhere to it. The hypothesis also assumed that the intervention group would display: positive changes in health; subjective variables such as HRQoL, laboratory values, and anthropometric dimensions; each of which would reduce the risk for the development of CVD.
Material and methods
Study design and recruitment procedure
This randomized controlled trial included patients with BRCA and CRC during adjuvant chemotherapy. Recruitment took place at three different oncology departments in Sweden and the patients were asked about participation by the physician or nurse when visiting the oncology clinic. At site A, n = 21 were asked to participate, at site B, n = 63 and at site C, n = 78. The study period was from November 2011 to May 2012. Participants were randomized to either the control or the intervention group by a ratio of 1:1. The randomization was done with stratification for each diagnostic group. The inclusion criteria were as follows: diagnosed with BRCA or CRC, stage 1–4, with ongoing adjuvant chemotherapy, and able to speak Swedish. The exclusion criterion was an inability to cope with the intervention (walking). Measurements were performed at baseline and post-intervention, and demographic, treatment and background data on lifestyle were collected through medical records and a questionnaire. The control group was given standard information on physical activity and had no restrictions placed on any of their physical activities.
The intervention
The intervention continued for 10 weeks and participants were encouraged to walk 10 000 steps each day, which is equivalent to approximately 8 km of daily walking, in line with the recommendation of guidelines from the Institute of Public Health in Sweden. The participants attended a supervised group walk, one hour per week over 10 weeks.
Data collection
Adherence to the intervention
Adherence to physical activity was measured using a pedometer (SILVA ex connect) to track the number of steps per day. The pedometer was placed on the hip of the patients. The pedometer included measures of distance, calories, a watch and USB port to transfer data to a computer. Data could be saved for up to a week in the pedometer. Participants received a self-report activity diary to record daily steps and the step count was reported weekly to the project manager. The electronic counts in the pedometers were not checked by the researchers; the reporting was the participant's responsibility. Adherence was assessed in two ways: by the number of participants who reported their actual step count every week; and the number of participants who achieved the target steps every week.
Health-related quality of life
Health-related quality of life (HRQoL) was assessed with the EORTC QLQ- C30 questionnaire [Citation25]; the EORTC QLQ-BR 23 [Citation26] for specific HRQoL issues relevant to patient with BRCA; and the EORTC QLQ-CR38 module for patients with CRC [Citation27].
Medical and demographic background data
Demographic data were collected through medical records and a study-specific questionnaire. Data included age, sex, performance status (WHO), cardiovascular comorbidity, educational level, marital status, employment, current exercise status, exercise behavior, and tobacco and alcohol use before the start of the intervention. Other medical information included cancer stage and type of chemotherapy treatment. Medical information at baseline and post-treatment included fasting laboratory assessment of fS-HDL, fS-LDL, fS-cholesterol, fS-triglycerides, and fS-C peptide. Body composition dimensions were collected from each patient and included weight, height, body mass index (BMI), abdominal circumference, abdominal height, blood pressure and resting heart rate.
Assessment of physical activity levels
Physical activity during the intervention was assessed by six project-specific questions rated on a six-level scale. The questions concerned how much the patients had been cycling and walking (rarely – ≥ 1.5 h per day), training (gym or aerobics; rarely – ≥ 5 h per week) and housework at home (< 1 h per day – ≥ 8 h per day). Further, participants were asked to rate their daily activity level (mostly sedentary – heavy labor) and how much time they spent reading and watching TV (< 1 h per day – ≥ 8 h per day). This questionnaire was study specific.
Statistical analyses
Statistical analyses were conducted using the software package SPSS version 20.0. Descriptive statistics were used to characterize the sample and study variables. T-tests were used for continuous variables and χ2 tests were used to determine categorical variables when testing differences between the intervention group and the control group. The effects of the intervention on body composition, blood samples and HRQoL assessment were analyzed by analysis of variance (ANOVA) with repeated measures. The questions concerning physical activity were summed to obtain a global measure of physical activity (after the responses regarding time spent for reading and watching TV had been reversed). Global physical activity was analyzed by ANOVA with repeated measures. In the EORTC QLQ-C30 results, substitution of missing values was made with the mean of each patient’s responses, provided that at least half of the subscale items had been completed. A statistical level of p = 0.05 was selected.
Ethics
The study was approved by the regional ethical review board in Uppsala Sweden (DI nr: 2011-321). Written informed concert was obtained from all patients prior to participation.
Results
Completion rate
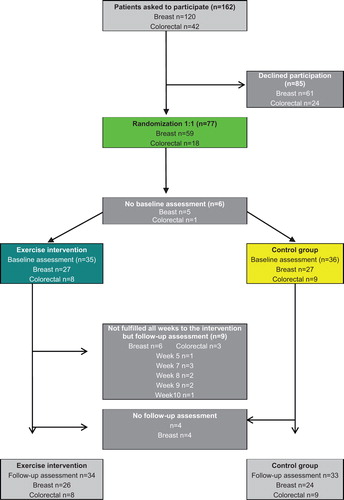
One hundred and sixty-two patients were asked to participate, 120 with BRCA and 42 with CRC. Of these, 77 agreed to participate and were randomized to either the intervention or control group. The most common reasons for non-participation were perceived stress during treatment, other health reasons, and fatigue. At the first baseline blood tests, six participants dropped out. During the study period four participants discontinued participation. Reasons for drop-outs were, for those who stated them; personal reasons, treatment side effects, stress (i.e. working full-time), fatigue or obesity. Finally, 67 patients (87%) completed the pre- and post- treatment assessment ().
Patient characteristics
The mean age of the participants was 54 years. The majority of participants were female; eight were men. More than half (51.9%) had a college or university degree and 62% were married or cohabiting. At baseline, 30% of the participants stated that they walked less than 20 minutes a day, and 30% stated they walked more than 40 minutes each day, while 61% reported that they almost never exercised by attending aerobics or the gym. The majority of the participants had received at least one chemotherapy treatment course at inclusion. No significant differences were found between the intervention and control groups in gender, performance status (WHO), cardiovascular co-morbidities and education level. Differences between the two groups were found for those patients with BRCA, mainly between age and smoking habits; the control group was slightly younger than the intervention group and the intervention group contained more non-smokers while the control group had more participants who had smoked but quit (). Some differences were observed at baseline in the exercise habits of the BRCA group where the intervention group had a slightly higher level of activity; also shows that BRCA participants in the intervention group generally were more physically active than the control group at baseline, while the situation was reversed in the CRC group, but it was not a significant difference ().
Table I. Patients’ demographics.
Table II. Global measures of physical activity. Participants exercise habits and changes over time. Sum variable, ANOVA with repeated measures.
Feasibility and adherence to the physical activity intervention
During the first four weeks of the intervention, adherence to patient participation for the total sample was 100%, and during the last four weeks the adherence rate was 81%. During the intervention period adherence averaged at 91%, and in total, 74% completed the physical activity intervention. Step count adherence towards the goal of 10 000 steps per day remained steady during the intervention period; with an average of about 8300 steps per day and an average of 34% of the participants managed to reach the step goal every week (). However, some patients were not able to walk due to adverse side effects but they were instead physically active with, e.g. swimming or biking; this data was not included in the analysis. The most common causes of impaired adherence of step registration were illness, difficulties with using the pedometer and experiencing adverse reactions to treatment. The intervention group showed a significant increase in their daily physical activity (p = 0.016) after 10 weeks compared with the control group ().
Table III. Adherence to the exercise intervention by percentage of participating patients, mean step per day, mean step per week and the percentage of participants who achieved the step goal each week.
Effects of the physical activity intervention during adjuvant chemotherapy treatment
HRQoL and symptoms
There were no significant differences in HRQoL between the intervention and control group for the colorectal sample (data not shown). In the BRCA group, breast symptoms such as swelling, mobility or pain around the operated breast significantly decreased for patients in the intervention group post-intervention, while the control group results were unchanged (p = 0.045).Other HRQoL data did not show any significant differences between control and interventions groups (data not shown). In terms of dyspnea, patients in the intervention group (BRCA) deteriorated less than the control group, however this result was not significant (p = 0.09). There were no differences between the intervention and control group among the three most commonly reported symptoms. Participants in the intervention group reported (in descending order) at baseline; insomnia, fatigue and dyspnea as being troublesome; whereas for the control group, fatigue ranked first then insomnia and dyspnea ().
Table IV. Effects of the exercise intervention on HRQoL and symptoms.
Body composition and laboratory values
There were no statistically significant differences found when comparing changes in blood samples and anthropometric data between the control and the intervention group at baseline and follow-up measures. The majority of the participants gained weight during the intervention period (p = 0.001) and the mean value for the weight gain was 0.9 kg in the intervention group and 1.3 kg in the control group, but no significant differences were found between groups (p = 0.528). There were no significant changes in abdominal circumference or abdominal height detected between the groups over time. Both groups showed slightly lower blood pressure, both systolic (p = 0.007) and diastolic (p = 0.027), during the study period and resting heart rate was unchanged ().
Table V. Anthropometric and laboratory values, ANOVA with repeated measures.
There were small changes in blood lipids over time for the total sample. Both the intervention and the control group had increased levels of fs-LDL and triglycerides during the study period but there were no differences between the two groups. C-peptide increased from baseline to post-measure (p = 0.09), an average of 0.4 mmol/L in the control group and 0.2 mmol/L in the intervention group, and there was no significant difference between the groups ().
Discussion
Adherence levels for the total sample in the study can be considered to be satisfactory. The majority of the participants achieved an average of 83% of the target of 10 000 steps per day for 10 weeks. The average adherence rate was high during the intervention period and the majority in the intervention group also managed to complete the 10 weeks intervention. As highlighted in earlier clinical trials [Citation24], involving patients in exercise intervention during cancer treatment has reported a wide range of adherence (26–93%); the results also point out that the reasons for poorer adherence rates seem to correlate to the prescribed dose of physical activity, implying that interventions with lower doses of physical activity show greater adherence rates than higher ones. Physical activity in the form of walking is often seen as a lower intensity exercise compared to supervised aerobic exercise sessions and may explain the relatively good adherence level shown in our study. However, in order to achieve 10 000 steps a day, approximately one hour and 30 minutes of physical activity is needed; this rate is, according to the WHO, a moderate level of physical activity [Citation28] during the intervention a third of the participants reached the goal, while the majority came close to the goal (83%). The adherence rates in the present study may also be related to the recruitment rate of 48%; one can speculate that those most interested in physical activities accepted inclusion which contributed to the high adherence. Generally, there are difficulties in achieving truthful response rates to adherence in studies, since often overall adherence rates are reported to the exercise intervention, rather than the number of participants who adhere to the stated goal, and this may lead to inappropriate conclusions [Citation23]. In the current study 26% [Citation9] of the participants did not manage to cope with all 10 weeks of the walking intervention and drop-outs most commonly occurred between weeks 7–9. Previous studies [Citation23,Citation24,Citation29] show that adherence to exercise interventions may be related to how often and how long the intervention period is; the longer the intervention, the harder it is for participants to cope over time. Another study [Citation29] also indicated that adherence correlates to specific time points between cycles; the more time that has passed since chemotherapy treatment, the more the adherence level increases. This information is important to consider when planning physical interventions during chemotherapy treatment in order to support and motivate patients to continue physical activity during treatment.
Our study shows that the participant adherence decreased the longer the duration of the study period and one explanation for this may be that participants simply did not report the steps towards the end of the study, despite several reminders. Different options for reporting were available for them: in person at the weekly walk, or by e-mail. Participants also reported a higher degree of fatigue post-measurement, and this could be because it is difficult to cope with being physically active and maintain the motivation as time goes by. However, there was no decrease in the percentage of achieved steps over time, which is not consistent with previous research results [Citation23,Citation24,Citation29] that show poorer adherence to the physical activity goal over time. One of the study limitations is the reliance on self-reported data, and the lack of electronic control of the reported steps. We have no way of being certain that the reported steps were accurate. Adherence levels during the intervention for the patients with CRC in our study were also generally lower than for patients with BRCA, except during the first week. Possible explanations for this result are that the CRC group reported poorer HRQoL at baseline compared to the BRCA patients. Patients with CRC report that they find it difficult to walk because they have symptoms like hand and foot syndrome as a side effect of treatment with xeloda and oxaliplatin. Other explanations for this are also that the CRC groups are older in age, and a higher proportion of patients with BRCA (53%) reported that they were more physically active than the CRC group (28%) at baseline. A limitation is that the instrument used to measure the activity level was developed for the study and not validated and was self-reported, which could be the reason why it was difficult to distinguish between before and after assessments of level of activity.
The study demonstrated one significant difference between the intervention and control groups of breast symptoms for patients with BRCA, but no other significant differences were found between the groups. In general, exercise studies have shown mixed results when it comes to the impact of physical activity on HRQoL. In rehabilitation studies with exercise, interventions after cancer treatment [Citation3,Citation9,Citation11] report more positive results on the perceived HRQoL compared with studies applying interventions during chemotherapy treatment [Citation9]. Several studies point out [Citation9,Citation21] that HRQoL as an outcome measure to assess exercise interventions is too broad to detect the likely effects and impact of the intervention on symptoms. A focus on more symptom-specific questionnaires may better cover these aspects. Positive results on HRQoL issues have been found for improved self-esteem [Citation9] and others have reported improvements in mood and less anxiety [Citation10] in patients who were physically active and improved their fitness levels during chemotherapy compared to those who were inactive. If there is a need for improvements in fitness to see significant outcomes in general HRQoL, a low impact walking activity may not be enough to alleviate the symptoms that are experienced during chemotherapy [Citation10] and that could be an explanation why this study reports few significant positive outcomes. However, a limitation is also the small sample size, which may have contributed to this result, and it is also possible that other more symptom-focused outcome measures may have contributed to better results. Another limitation in the study is that several participants had undergone a different number of previous chemotherapy treatments at the point of their inclusion; this can obviously affect the assessment of HRQoL and lifestyle questions, because patients were not all in the same phase of treatment.
The control group, on average, gained a little more weight (1.3 kg) than the intervention group (0.9 kg). Weight gain in our study was relatively small and can be seen as a positive result in comparison with previous studies with adjuvant chemotherapy for BRCA [Citation15]. Research has also found that weight loss during exercise intervention in patients undergoing chemotherapy is unusual [Citation4,Citation9]. The majority of studies with positive results have demonstrated sustained weight and body composition and the weight that patients gain during treatment has also been found to be maintained after treatment [Citation15], therefore it is important to prevent weight gain during adjuvant chemotherapy.
A statistically significant decrease in blood pressure was observed in both the control and intervention group, both systolic (p = 0.007) and diastolic (p = 0.027), but no differences were found between the two groups. This result may be explained by the fact that the participants might have been active enough before the start of treatment, and thus, this type of intervention did not affect their blood pressure rates. A systematic review that evaluated the effect on patients who used pedometers and their blood pressure change, showed that an increased number of steps correlated to a reduction in systolic blood pressure, and that the magnitude of lower blood pressure was related to the increased number of steps per day [Citation30]. It is difficult to find comparative data for blood pressure because this is not a common variable assessed in low impact physical activity trials for patients with cancer [Citation10,Citation31].
No statistically significant difference between the groups was found for cholesterol or c-peptide. Cholesterol levels increased overall in the sample; however the increase was smaller in the intervention group but not significantly. For the c-peptide results, the increase was higher in the control group than the intervention group; similar result have also been shown in other studies including patients with cancer [Citation32].
Conclusion
A low intensity physical activity intervention in the form of walking is feasible during adjuvant chemotherapy treatment. It is possible to walk, despite increasing symptoms and distress walking seems to be a feasible alternative. Despite the small number of participants taking part in this pilot study, some positive impact on breast symptoms was reported. The study also showed a relatively small weight gain in comparison to other studies. It is important to emphasize that high adherence to low impact physical activity during treatment does not naturally lead to improvements in health. This study reports few significant differences between the groups in health-related variables. Possible actions to improve outcomes for patients with BRCA and CRC during chemotherapy treatment may be: higher intensity of the physical activity intervention such as supervised exercise including individualized programs; the inclusion of more objective measures to evaluate performance; and larger samples to enhance the value of physical activity interventions.
Acknowledgments
We wish to acknowledge Daniel Brattström MD and Anna Öberg-Wrangsjö MD for participating in the data collection and clinical management of this study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Barbaric M, Brooks E, Moore L, Cheifetz O. Effects of physical activity on cancer survival: A systematic review. Physiother Can 2010;62:25–34.
- Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: A review of the literature. Br J Cancer 2011;105(Suppl 1):S52–73.
- Denlinger CS, Engstrom PF. Colorectal cancer survivorship: Movement matters. Cancer Prev Res 2011;4:502–11.
- Markes M, Brockow T, Resch KL. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev 2006;(4): CD005001.
- Jones LW, Alfano CM. Exercise-oncology research: Past, present, and future. Acta Oncol 2013;52:195–215.
- Oh HS, Seo WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldv Evid-based Nu 2011;8:191–201.
- Montazeri A. Health-related quality of life in breast cancer patients: A bibliographic review of the literature from 1974 to 2007. J Experiment Clin Cancer Res 2008;27:32.
- Jansen L, Koch L, Brenner H, Arndt V. Quality of life among long-term (>/ = 5 years) colorectal cancer survivors – systematic review. Eur J Cancer 2010;46:2879–88.
- Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol 2007;25:4396–404.
- Yang CY, Tsai JC, Huang YC, Lin CC. Effects of a home-based walking program on perceived symptom and mood status in postoperative breast cancer women receiving adjuvant chemotherapy. J Adv Nurs 2011;67:158–68.
- Carmichael AR, Daley AJ, Rea DW, Bowden SJ. Physical activity and breast cancer outcome: A brief review of evidence, current practice and future direction. Eur J Surg Oncol 2010;36:1139–48.
- Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J Cancer Surviv 2010;4:87–100.
- Meyerhardt JA. Beyond standard adjuvant therapy for colon cancer: Role of nonstandard interventions. Semin Oncol 2011;38:533–41.
- McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J Clin Oncol 2010;28:4074–80.
- Gadea E, Thivat E, Planchat E, Morio B, Durando X. Importance of metabolic changes induced by chemotherapy on prognosis of early-stage breast cancer patients: A review of potential mechanisms. Obes Rev 2012;13:368–80.
- Chowdhury TA. Diabetes and cancer. QJM 2010;103: 905–15.
- Irwin ML, Duggan C, Wang CY, Smith AW, McTiernan A, Baumgartner RN, et al. Fasting C-peptide levels and death resulting from all causes and breast cancer: The health, eating, activity, and lifestyle study. J Clin Oncol 2011;29: 47–53.
- Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 2008;299:1261–3.
- Dunn AL. The effectiveness of lifestyle physical activity interventions to reduce cardiovascular disease. Am J Lifestyle Med 2009;3:11S–8S.
- Winzer BM, Whiteman DC, Reeves MM, Paratz JD. Physical activity and cancer prevention: A systematic review of clinical trials. Cancer Cause Control 2011;22:811–26.
- Demark-Wahnefried W, Case LD, Blackwell K, Marcom PK, Kraus W, Aziz N, et al. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin Breast Cancer 2008;8:70–9.
- Bertheussen GF, Kaasa S, Hokstad A, Sandmael JA, Helbostad JL, Salvesen O, et al. Feasibility and changes in symptoms and functioning following inpatient cancer rehabilitation. Acta Oncol 2012;51:1070–80.
- Daley AJ, Crank H, Mutrie N, Saxton JM, Coleman R. Determinants of adherence to exercise in women treated for breast cancer. Eur J Oncol Nurs 2007;11:392–9.
- Carayol M, Bernard P, Boiche J, Riou F, Mercier B, Cousson-Gelie F, et al. Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: What is the optimal dose needed?Ann Oncol 2013;24:291–300.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality- of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Sprangers MA, Groenvold M, Arraras JI, Franklin J, te Velde A, Muller M, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. J Clin Oncol 1996;14:2756–68.
- Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer 1999;35:238–47.
- Global rekomendations on physical activity for health. [cited 2013 Aug 20]. Available from: http://www.who.int/dietphysicalactivity/factsheet recommendations/index.html
- Swenson KK, Nissen MJ, Henly SJ. Physical activity in women receiving chemotherapy for breast cancer: Adherence to a walking intervention. Oncol Nurs Forum 2010;37: 321–30.
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: A systematic review. JAMA 2007;298:2296–304.
- Wang YJ, Boehmke M, Wu YW, Dickerson SS, Fisher N. Effects of a 6-week walking program on Taiwanese women newly diagnosed with early-stage breast cancer. Cancer Nurs 2011;34:E1–13.
- Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, et al. Relationship of obesity and physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomark Prev 2005;14:2881–8.