Abstract
Background. Surgery followed by radiotherapy and concomitant and adjuvant temozolomide is standard therapy in newly diagnosed glioblastoma multiforme (GBM). Bevacizumab combined with irinotecan produces impressive response rates in recurrent GBM. In a randomized phase II study, we investigated the efficacy of neoadjuvant bevacizumab combined with irinotecan (Bev-Iri) versus bevacizumab combined with temozolomide (Bev-Tem) before, during and after radiotherapy in newly diagnosed GBM.
Material and methods. After surgery, patients were randomized to Bev-Iri or Bev-Tem for eight weeks, followed by standard radiotherapy (60 Gy/30 fractions) and concomitant Bev-Iri or Bev-Tem followed by adjuvant Bev-Iri or Bev-Tem for another eight weeks. Bev-Iri: Bevacizumab and irinotecan were given every 14 days before, during and after radiotherapy. Bev-Tem: Bevacizumab was given as in Bev-Iri and temozolomide was given for five days every four weeks before and after radiotherapy and once daily during radiotherapy. The primary endpoint was response after neoadjuvant chemotherapy and a pre-specified response rate of 30% or more was considered of interest for future studies. Secondary endpoints were progression-free survival (PFS) and toxicity.
Results. The response rate was 32% (95% CI 17–51%) for Bev-Tem (n = 32) and 23% (95% CI 9–44%) for Bev-Iri (n = 31) (p = 0.56). Median PFS was 7.7 and 7.3 months for Bev-Tem and Bev-Iri, respectively. Hematological toxicity was more frequent with Bev-Tem including one death from febrile neutropenia whereas non-hematological toxicity was manageable.
Conclusions. Only the Bev-Tem arm met the pre-specified level of activity of interest. Our results did not indicate any benefit from Bev-Iri in first-line therapy as opposed to Bev-Tem in terms of response and PFS.
Glioblastoma multiforme (GBM) is a highly angiogenic tumor with upregulated vascular endothelial growth factor (VEGF). VEGF-A activity is rate-limiting in blood vessel growth [Citation1] and bevacizumab inhibits angiogenesis by sequestering VEGF-A [Citation2]. In mice, a murine anti-human VEGF monoclonal antibody inhibited human tumor growth and increased efficacy combined with chemotherapy [Citation3]. Bevacizumab has been approved in the EU for a number of malignancies, e.g. colorectal and ovarian cancer.
Irinotecan, a topoisomerase-I inhibitor, crosses the blood-brain barrier and has achieved response rates from 10% to 15% in recurrent GBM; however, irinotecan and bevacizumab in combination in recurrent GBM produced response rates from 25% to 57% in phase II studies [Citation4–9]. The response rate in recurrent GBM following bevacizumab monotherapy was 28% in the phase II study AVF3708g [Citation9] and 35% in the phase II study NCI 06-C-0064E [Citation10], leading to FDA approval of bevacizumab monotherapy in second-line GBM.
Temozolomide is used in GBM in first-line therapy combined with radiotherapy and in recurrent disease. Temozolomide combined with bevacizumab was safe and exhibited significant activity in both first- and second-line therapy [Citation11,Citation12].
At the time of the design of this study, two large randomized phase III studies in GBM were launched (the RTOG 0825 study and the Avaglio study), investigating bevacizumab added to standard first-line treatment; however, considering the impressive phase II response data, we hypothesized that bevacizumab and irinotecan and concurrent radiotherapy as first-line therapy may be a strategy to improve the prognosis in GBM. We assessed the response rate of bevacizumab and irinotecan or bevacizumab and temozolomide in a randomized phase II study and this neoadjuvant treatment design enabled assessment of response independent of subsequent radiotherapy. The study is exploratory and significant activity in the irinotecan arm would support future studies with bevacizumab and irinotecan in the first-line treatment of GBM.
Methods
Design
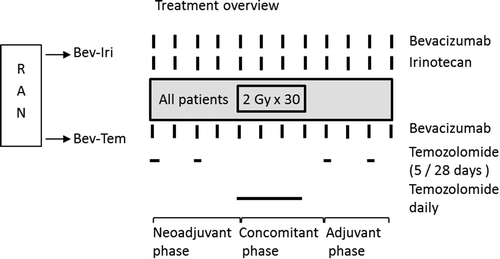
Patients were randomized to bevacizumab-irinotecan (Bev-Iri) or bevacizumab-temozolomide (Bev-Tem) for eight weeks, followed by radiotherapy and concomitant Bev-Iri or Bev-Tem. Following the concomitant chemoradiotherapy, adjuvant Bev-Iri or Bev-Tem was continued for another eight weeks (). Continued adjuvant treatment hereafter was offered to non-progressing patients that tolerated the treatment at the discretion of the treating physician. In case of disease progression, cross-over to the alternative regimen was allowed. The primary endpoint was response rate after eight weeks of neoadjuvant chemotherapy. Secondary endpoints were progression-free survival (PFS) and toxicity. Response was assessed every eight weeks and toxicity was assessed every two weeks. A central computer-based randomization was performed by the head of data management at the clinical research facility at Rigshospitalet, blinded to the patient data. Randomization was stratified by center. The study was approved by the Danish Health and Medicines Authority, the regional ethics committee and the local review boards in all participating centers. The study was registered at clinicaltrials. gov as NCT-00817284.
Treatment
Bevacizumab 10 mg/kg was administered every two weeks for the full duration of the study (before, during and after radiotherapy) in both treatment arms. Irinotecan was similarly administered every two weeks for the full duration of the study: Patients on enzyme inducing anti-epileptic drugs (EIAID) received a dose of 340 mg/m2 while patients not on EIAIDs received a dose of 125 mg/m2. Temozolomide 200 mg/m2 was given daily for five days followed by 23 days off, before and after the radiotherapy. During the radiotherapy, a dose of temozolomide of 75 mg/m2 was given daily. Due to the risk of post-operative complications from bevacizumab, treatment was not started until ≥ 4 weeks from the initial surgery or primary biopsy.
For radiotherapy planning, a cerebral computed tomography (CT) scan was done with 3 mm slices. This CT scan, the baseline magnetic resonance (MR) scan and the MR scan after neoadjuvant therapy, was used for radiotherapy planning. In case of tumor growth at eight weeks, prior to the start of radiotherapy, the largest target was used for planning of radiotherapy. The gross tumor volume (GTV) was defined as contrast enhancing tumor on T1-weighted MR scans and the surgical cavity if present. The clinical target volume (CTV) was defined as the GTV + 2 cm margin, except for bony structures. Meningeal structures were considered anatomic barriers to tumor spread. No ITV was defined. No variations in size, shape or position of CTV in relation to anatomical reference structures were considered. The planning target volume (PTV) was defined as the CTV + 0.5 cm margin. Radiotherapy was 60 Gy to the PTV in 30 fractions/6 weeks by a megavoltage linear accelerator (6 MeV), by conformal three- dimensional (3D) three-field technique or Rapid Arc. Tolerance doses were as by Emami et al. [Citation13].
Inclusion criteria
Histological verified GBM; residual contrast enhancing tumor ≥ 1 cm on the baseline MR scan (done ≤ 14 days prior to therapy); informed consent; no prior therapy for GBM except resection or biopsy; performance status ≤ 2; age ≥ 18 years; platelets, hemoglobin, leukocytes and neutrophile granulocytes within normal limits, liver transaminases ≤ 3 × upper normal limit (UNL), bilirubin ≤ 1.5 × ULN, se-creatinin ≤ 1.5 × UNL or a glomerular filtration rate > 60 ml/min by Cr-EDTA clearance, activated partial thromboplastin time and international normalized ratio ≤ UNL; use of oral contraceptives or IUD by fertile females and use of condom by fertile males; no cerebral bleeding on baseline MR scan.
Exclusion criteria
Medication or other conditions that may affect interpretation of results; previous cancer ≤ 5 years except adequately treated basal/squamous cell skin cancer or cervical carcinoma in situ; heart disease > NYHA class II, significant cardiac arrhythmia, unstable angina pectoris or myocardial infarction ≤ 6 months; significant peripheral arterial disease; use of acetylsalicylic acid, non-steroid anti-inflammatory drugs or clopidogrel; major surgery or open biopsy < 28 days prior to therapy; fine-needle biopsy < 7 days prior to therapy; gastrointestinal fistulas, perforations or abscesses < 6 months; chronic inflammatory intestinal disease; HIV/Hepatitis B/C infection; uncontrolled infection or diabetes mellitus; non-healing ulcers ≤ 6 months; bone fractures ≤ 3 months; pregnancy or lactation; blood pressure> 150 systolic or > 100 diastolic mmHg (antihypertensive medication was allowed); proteinuria ≥ 1 g/day; allergy to irinotecan, temozolomide or bevacizumab.
Evaluation of efficacy
For the evaluation of the primary endpoint, all MR scans were evaluated blinded by an expert in neuroradiology (CT). Response was evaluated according to modified MacDonald criteria. CR: Complete disappearance of all contrast enhancing disease and T2-changes, no new lesions, no use of corticosteroids and no decrease in clinical status. PR: Decrease ≥ 50% in the sum of the products of the perpendicular diameters of all measurable contrast enhancing lesions, no new lesions, no increase in T2-changes, no increase in corticosteroid dose and no decrease in clinical status. Progressive disease (PD): Increase ≥ 25% in the sum of the products of perpendicular diameters of contrast enhancing lesions, and/or the appearance of new contrast enhancing lesions, and/or a significant increase in dose of corticosteroids and/or significant clinical deterioration. To be able to quantitate efficacy in more detail, we further defined minor response (mPR) as a decrease of 25–50% of the product of perpendicular diameters of all measurable contrast enhancing lesions, no new lesions, no increase in corticosteroid dose and no decrease in clinical status. Stable disease (SD) was declared if the patient did not qualify for CR, PR, mPR or PD. Contrast enhancing lesions less than 10 mm in diameter at baseline were defined as evaluable but not measurable.
Within three months following completion of chemoradiotherapy, radiological PD was only declared if this could be confirmed on a subsequent MR scan with an interval of eight weeks; if radiological PD was not confirmed, pseudoprogression (PsPD) was declared unless there was clinical deterioration.
The PFS and overall survival (OS) were determined in the per-protocol population, including the patients with non-measurable disease at baseline. If a patient had been declared radiological PD during the study but this could not be confirmed in the blinded review, the patient was censored for PFS at the time of the investigator determined radiological PD; however, patients were not censored in case of clinical progression. Patients were censored for OS if they were alive at the date of analysis.
Statistics
This study was considered as two parallel phase II studies and Simon's optimal two-stage design was applied to estimate sample size for each arm. A response rate of ≥ 30% was considered worthy of further study, whereas a response rate of < 10% was considered inactive. A risk of 5% (alpha) for recommending an inactive treatment arm for further study and a risk of 20% (beta) for rejecting a potentially active treatment were accepted. Under these assumptions a treatment arm required > 5 of 29 responders. In addition, an early futility analysis was performed after 10 evaluable patients. The study should be terminated if ≤ 1 response was observed among the first 10 patients. If ≥ 2 responses were observed the inclusion would continue up to 29 evaulable patients. The Kaplan-Meier method was used to analyze time-to-event data.
Results
Enrollment
A total of 65 patients, the intention-to-treat population, were randomized from November 2008 to November 2010 at three regional oncology centers in Denmark. Two randomized patients were not treated and were not included in the per-protocol analysis: One patient was claustrophobic and refused the MR scan and one had a cerebral bleeding on the baseline MR scan. According to the study protocol, patients that went off-study prior to the chemoradiation phase were replaced by new patients. Three patients progressed prior to the chemoradiation phase and were replaced during the study; however, these three patients were included in the per-protocol population that thus consisted of 31 patients on the Bev-Iri arm and 32 patients on the Bev-Tem arm.
Baseline characteristics
The baseline characteristics, mini-mental state examination (MMSE) score and steroid dose were well balanced. Five patients were biopsied only; the rest had a partial resection ().
Table I. Patient characteristics.
Response
Two patients had too poor quality baseline MR scan and four patients did not have contrast enhancing tumor on baseline MR scan as determined by the blinded review. These six patients were excluded from the response analysis. There were no complete responses. The partial response rate in the Bev-Tem arm was 32% (95% CI 17–51%) and in the Bev-Iri arm it was 23% (95% CI 9–44%) (p = 0.56). The mPR was 35.5% (95% CI 19–55%) in the Bev-Tem arm and 27% (95% CI 12–48%) in the Bev-Iri arm (p = 0.57) and the combined partial and minor response rate in the Bev-Tem arm was 68% (95% CI 49–83%) and 50% (95% CI 29–70%) in the Bev-Iri arm (p = 0.19). The frequency of PD at eight weeks in the per-protocol population (n = 63) was 13% in the Bev-Tem arm (95% CI 4–29%) compared to 19% in the Bev-Iri arm (95% CI 8–38%) (p = 0.73) ().
Table II. Response after neoadjuvant chemotherapy.
Progression-free survival and survival
The median follow-up was 31 months (range: 19–43 months). In the per-protocol population, the median PFS from Bev-Iri was 7.3 months (95% CI 5.0–9.3 months) and 7.7 months for Bev-Tem (95% CI 5.1–10.2 months) (p = 0.79). Similarly, the six- and 12-months PFS were 52% and 10% in the Bev-Iri arm and 53% and 6% in the Bev-Tem arm, respectively (data not shown). The median survival in the full per-protocol population (n = 63) was 13.8 months (95% CI 12.4–15.3 months). The median survival was 15.1 months in the Bev-Iri arm (95% CI 9.6–20.6 months) and 11.8 months in the Bev-Tem arm (95% CI 8.2–15.3 months).
Safety
The non-hematological toxicity from the neoadjuvant therapy was mostly modest with no wound dehiscence, CNS bleeding or ischemia. Besides alopecia, non-hematological toxicity was very similar in the two arms (). In all six cycles combined there were 128 episodes of grade I/II and 12 episodes of grade III/IV non-hematological toxicities on the Bev-Tem arm compared to 135 and 11 on the Bev-Iri arm. No unexpected toxicity was observed from bevacizumab, chemotherapy and concomitant radiotherapy.
Table III. Non-hematological toxicity from neoadjuvant treatment.
Hematological toxicity was more frequent on the Bev-Tem arm with eight episodes of III/IV hematological toxicities during the neoadjuvant phase compared to three episodes from Bev-Iri (): In all six cycles combined there were 22 episodes of grade III/IV hematological toxicities including one case of fatal febrile neutropenia (grade V toxicity) from Bev-Tem, compared to eight episodes of grade III/IV toxicity from Bev-Iri.
Table IV. Hematological toxicity from neoadjuvant treatment.
Discussion
The response rate (PR + CR) was 32% in the Bev-Tem arm compared to 23% in the Bev-Iri arm and correspondingly, the mPR was 36% and 27%; PR and mPR combined was 68% in the Bev-Tem arm and 50% in the Bev-Iri arm (all differences were non-significant). Previous response rates from neoadjuvant temozolomide in GBM were 41% in 36 patients [Citation14] and 52% in 33 patients [Citation15] whereas in a study in 162 patients it was only 4% [Citation16]. These discrepancies may be due to differences in patient population and to differences in interpretation of radiological response.
Pseudoprogression following chemoradiotherapy may complicate response evaluation but our assessments were done prior to radiotherapy and were therefore not susceptible to this misinterpretation. However, pseudoresponse reflecting rapid bevacizumab induced changes on MR scans, may reflect rapid alterations in vessel permeability, rather than clonogenic cell death and real tumor shrinkage. Our assessments were done eight weeks from start of therapy and it was therefore thought that the initial rapid appearance of pseudoresponse was less likely to interfere with interpretation [Citation17]. However, we cannot rule out pseudoresponse and this is a potential source of error.
Our response rate of 32% in the Bev-Tem arm is similar to the studies discussed above and to those obtained from bevacizumab monotherapy [Citation9,Citation10]. The results also compare well with bevacizumab and irinotecan in the recurrent setting [Citation4–9] and compare favorably with those reported by Brada et al. [Citation16]. Comparison of responses between first- and second-line therapy should be interpreted with caution however.
In a phase II study, PFS, but not survival, was improved with bevacizumab added to standard first-line therapy in newly diagnosed GBM compared to historical controls [Citation12]. Similarly, PFS was prolonged from 6.2 months to 10.6 months in the AVAGlio study [Citation18] and from 7.3 months to 10.7 months in the RTOG0825 study, without improvement of OS [Citation19].
Methylation of the MGMT gene is associated with improved outcome following radiotherapy and standard concurrent and adjuvant temozolomide when measured by methylation-specific polymerase reaction (PCR) [Citation20] and interestingly the six-months PFS in patients with newly diagnosed GBM with MGMT non-methylated tumors was significantly improved from 26% after radiotherapy and standard concurrent and adjuvant temozolomide to 71% after first-line therapy with radiotherapy, bevacizumab and irinotecan [Citation21]. Currently, we do not have access to PCR data, and this may weaken our conclusions.
In our per-protocol population (n = 63) the median survival was 13.8 months, and in the Bev-Tem arm it was 11.8 months. This is inferior to the 14.6 months reported by Stupp et al. [Citation22] and the 16.6 months in the RTOG0525 study following standard chemoradiation [Citation23]. A reason may be that our patients were only biopsied or incompletely resected and had measurable contrast enhancing residual tumor and this may represent worse prognosis patients. Another reason could be the delay of the concurrent chemoradiation.
Toxicity in our study was manageable and is comparable with data by Vredenburgh [Citation24] and Narayana [Citation25] whereas Lai et al. [Citation12] observed a number of serious adverse events, e.g. cerebrovascular and gastrointestinal complications.
When designing this study, we speculated that Bev-Iri was more efficacious compared to Bev-Tem and if confirmed, further clinical investigation of first-line irinotecan and bevacizumab would be supported. However, neither response nor PFS was improved from Bev-Iri. In conclusion, our data do not indicate a benefit from irinotecan in first-line therapy, compared to temozolomide.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by a research grant and supply of bevacizumab by F. Hoffman La Roche. This clinical study was performed in compliance with current law in Denmark. Steinbjørn Hansen and Henrik P. Schultz are advisors for F. Hoffman La Roche and Hans Skovgaard Poulsen has been in an advisory board meeting for F. Hoffman La Roche. Ulrik Lassen has received funding for biomarker studies in inhibition of VEGF and has received a one-time speaker's fee.
References
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–76.
- Reardon DA, Turner S, Peters KB, Desjardins A, Gururangan S, Sampson JH, et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw 2011;9:414–27.
- Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 2005;65:671–80.
- Moller S, Grunnet K, Hansen S, Schultz H, Holmberg M, Sorensen M, et al. A phase II trial with bevacizumab and irinotecan for patients with primary brain tumors and progression after standard therapy. Acta Oncol 2012;51: 797–804.
- Stark-Vance V. Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma. Neuro-Oncol 2005;7:369.
- Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007; 25:4722–9.
- Poulsen HS, Grunnet K, Sorensen M, Olsen P, Hasselbalch B, Nelausen K, et al. Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol 2009; 48:52–8.
- Hasselbalch B, Lassen U, Hansen S, Holmberg M, Sorensen M, Kosteljanetz M, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: A phase II trial. Neuro Oncol 2010;12:508–16.
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733–40.
- Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009; 27:740–5.
- Vredenburgh JJ, Desjardins A, Kirkpatrick JP, Reardon DA, Peters KB, Herndon JE, et al. Addition of bevacizumab to standard radiation therapy and daily temozolomide is associated with minimal toxicity in newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys 2012;82:58–66.
- Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 2011;29:142–8.
- Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–22.
- Gilbert MR, Friedman HS, Kuttesch JF, Prados MD, Olson JJ, Reaman GH, et al. A phase II study of temozolomide in patients with newly diagnosed supratentorial malignant glioma before radiation therapy. Neuro Oncol 2002; 4:261–7.
- Friedman HS, McLendon RE, Kerby T, Dugan M, Bigner SH, Henry AJ, et al. DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis and response to Temodal in newly diagnosed malignant glioma. J Clin Oncol 1998;16:3851–7.
- Brada M, Ashley S, Dowe A, Gonsalves A, Huchet A, Pesce G, et al. Neoadjuvant phase II multicentre study of new agents in patients with malignant glioma after minimal surgery. Report of a cohort of 187 patients treated with temozolomide. Ann Oncol 2005;16:942–9.
- Hygino da Cruz LC, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: Imaging challenges in the assessment of posttreatment glioma. Am J Neuroradiology 2011;32:1978–85.
- Chinot O, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Phase III trial of bevacizumab added to standard radiotherapy and temozolomide for newly- diagnosed glioblasoma: Mature progression-free survival and preliminary overall survival results in Avaglio. Neuro-Oncology 2012;14(Suppl 6):101–5.
- Gilbert MR, Dignam J, Won M, Blumenthal DT, Vogelbaum MA, Aldape KD, et al. RTOG 0825: Phase III double-blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM) [abstract]. J Clin Oncol ASCO Annual Meeting Proceedings 2013;31:1.
- Olson RA, Brastianos PK, Palma DA. Prognostic and predictive value of epigenetic silencing of MGMT in patients with high grade gliomas: A systematic review and meta-analysis. J Neurooncol 2011;105:325–35.
- Herrlinger U, Schaefer N, Steinbach JP, Weyerbrock A, Hau P, Goldbrunner R, et al. Bevacizumab, irinotecan, and radiotherapy versus standard temozolomide and radiotherapy in newly diagnosed, MGMT-non-methylated glioblastoma patients: First results from the randomized multicenter GLARIUS trial [abstract]. J Clin Oncol ASCO Annual Meeting Proceedings 2013;31: LBA2000.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96.
- Gilbert M.R, Wang M, Aldape KD, Stupp R, Hegi M, Jaeckle KA, et al. RTOG 0525: A randomized phase III trial comparing standard adjuvant temozolomide (TMZ) with a dose-dense (dd) schedule in newly diagnosed glioblastoma (GBM) [abstract]. J Clin Oncol ASCO Annual Meeting Proceedings 2011:29:2006.
- Vredenburgh JJ, Desjardins A, Reardon DA, Peters KB, Herndon JE, Marcello J, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res 2011;15;4119–24.
- Narayana A, Gruber D, Kunnakkat S, Golfinos JG, Parker E, Raza S, et al. A clinical trial of bevacizumab, temozolomide, and radiation for newly diagnosed glioblastoma. J Neurosurg 2012;116:341–5.