Abstract
Purpose. To explore the relationship between radiotherapy (RT) dose levels in the arm/shoulder region and arm/shoulder morbidity in breast cancer patients.
Material and methods. This study included 183 breast cancer patients who had received locoregional RT with or without chemotherapy and/or hormone treatment during the period 1998–2002. Individual RT dose level, reflected by dose-volume histograms (DVHs), for the shoulder joint and joining structures were obtained from archived CT-based RT plans. Individual median, mean and maximum arm/shoulder RT dose levels were extracted. Arm/shoulder morbidity was assessed 29–58 months after breast cancer treatment using the following clinical endpoints: arm pain, arm stiffness, swollen arm, use of arm, numbness, shoulder flexion and shoulder abduction difference, fibrosis and breast cancer-related lymphedema. The relationship between arm/shoulder RT dose level and these clinical endpoints was assessed by Spearman's correlation and multivariate logistic regression.
Results. Ninety-one percent of the included patients had some degree of arm/shoulder morbidity. Neither mean nor maximum RT dose level was associated with clinical endpoints. However, significant correlations (p < 0.05) were found between DVHs and arm stiffness, arm pain, use of arm and shoulder abduction difference, when arm/shoulder RT dose levels were approximately 15 Gy.
Conclusions. Three-dimensional conformal locoregional RT for breast cancer results in long-term arm/shoulder morbidity. To minimize this risk, large shoulder volumes receiving RT doses of approximately 15 Gy should be reduced.
Breast cancer is the most frequent malignant disease among women worldwide, with an estimated 1.4 million new cases per year and about 460 000 deaths per year [Citation1]. It is well established that post-operative radiotherapy (RT) reduces 10-year locoregional recurrence rates and the 15-year risk of breast cancer death by 15.7% and 3.8%, respectively [Citation2]. RT may also increase long-term survival of patients who present with early-stage breast cancer [Citation3–5].
Breast cancer treatment may lead to side effects such as arm/shoulder pain, restricted arm/shoulder mobility, fibrosis and breast cancer-related lymphedema (BCRL). RT has been identified as a main cause of arm pain among breast cancer patients [Citation6–12]. In addition to mastectomy and axillary dissection, RT has also been consistently reported as a risk factor for BCRL [Citation13]. Adjuvant RT may add to the risk of restricted arm/shoulder mobility and post-operative BCRL [Citation14]. The reported prevalence of restricted arm/shoulder mobility varies considerably in breast cancer patients receiving RT, depending on the overall treatment plan applied, and the method of assessment [Citation15]. Furthermore, subcutaneous fibrosis has been identified as a risk factor for shoulder morbidity among patients receiving post-mastectomy RT [Citation16].
The recent introduction of and improvement in new surgical and radiotherapeutical procedures, such as sentinel lymph node biopsy or CT-based RT planning, is expected to result in a decrease in arm/shoulder morbidity among breast cancer patients, but it will not eliminate morbidity completely. Therefore, in order to minimize these adverse effects it is still vital to explore the relationship between RT and arm/shoulder morbidity. This study aims to investigate the relationship between arm/shoulder RT dose levels and arm/shoulder morbidity in breast cancer patients whose underwent CT-based RT.
Patients and methods
Patients and study design
In 2003/2004, 415 breast cancer patients treated from 1998 to 2002 with locoregional RT, with or without chemotherapy and/or hormone treatment at the Norwegian Radium Hospital, Oslo University Hospital (NRH-OUS) were invited to take part in a long-term follow-up study [Citation11]. All patients who had undergone surgery for stage II or III breast cancer, consisting of modified radical mastectomy or breast conserving surgery and axillary node dissection (level I or II), were invited. A total of 143 patients had undergone systemic chemotherapy (FEC = 5fluoro-uracil, epirubicin, cyclophosfamide) prior to RT.
To participate, patients had to fulfil the following inclusion criteria: 1) application of adjuvant RT to the chest wall and regional lymph nodes; 2) age ≤ 75 years in 2004; 3) no recurrence of breast cancer; and 4) no other invasive cancer except basal cell carcinoma, or prior or simultaneous contralateral breast cancer stage I treated with surgery only. The study consisted of a mailed questionnaire and an out-patient examination at the NRH-OUS. Of the 415 invited patients, 318 (77%) completed the questionnaire and attended the out-patient examination. The current study is restricted to the 183 patients who underwent CT-based RT and attended the out-patient examination (). Radiation exposure from modern conformal RT was based on a detailed dose-volume histogram (DVH) analysis.
Table I. Patient demographics and treatment-related characteristics for 183 included breast cancer patients.
All participants provided written informed consent, and the study was approved by the Ethical committee of the South-Eastern Norway Health authority and the Data Inspectorate of Norway.
Radiotherapy
All women were treated with four-field RT in which the target volume included all of the following: the breast (after breast conserving surgery) or the chest wall (after modified radical mastectomy), the ipsilateral supra-and infraclavicular fossa, ipsilateral lymph nodes along the internal mammary artery and ipsilateral axilla (). CT-based RT planning was carried out using CT scans that covered the region from the 6th cervical vertebra to the middle part of the abdomen. The CT slice thickness was 1.0 cm. The clinical target and treatment volumes, as well as both lungs and the heart, were routinely delineated in the CT images. CT-based RT planning and dose calculation were performed using the Helax-TMS (Version 6.0 or higher) system.
Figure 1. A. Schematic display of the four-field arrangement used in CT-RT. The red field borders depict the anterior-posterior field (1), the pink color indicated the oblique filed (2), and the blue color illustrates the location of the tangential fields (3 and 4). B. Delineated shoulder joint on the planning CT-images.
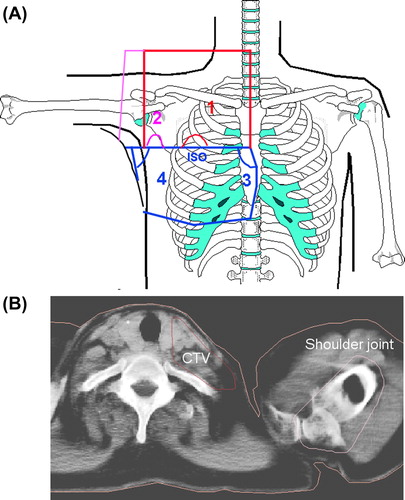
In 1998 and during most of 1999, all patients with metastatic axillary lymph nodes received RT to the entire axilla. Since the end of 1999 patients who had ≥ 10 axillary nodes removed received RT only to the axillary apex. Patients with < 10 axillary nodes removed received RT to the entire axilla.
The beam arrangement consisted of four half-beams with two tangential beams covering the caudal part of the target volume, one anterior- posterior field (0°) and one oblique field, typically 110–115°, covering the cranial part of the chest wall (). Fields 1 and 2 were used to treat the ipsilateral supra- and infraclavicular, axillary and internal mammary lymph nodes and the cranial part of the breast/breast wall, while tangential fields 3 and 4 covered the caudal part of the breast/ breast wall. The photon beam energy was mainly 6 MV. The dose plans were normalized to the mean dose provided to the target volume. The breast/chest wall received a total dose of 50 Gy and the regional lymph nodes 46–50 Gy, given in 2 Gy per day, five days per week. Thirty-six lumpectomized women, all younger than 50 years of age, received a boost of 10 or 16 Gy to the tumor bed using 9–12 MeV electron beams with a diameter of 5–9 cm. In mastectomized women with stage III tumors, or those with stage II tumors with tumor-positive resection margins, a bolus was used that covered the whole breast/chest wall; otherwise a 6 cm wide bolus covered the mastectomy scar only.
Shoulder volume definition
For the purpose of the study one radiologist delineated the shoulder volume, taking into account the limitations of CT imaging regarding soft-tissue structures (). The shoulder volume in each CT slice was defined by the identification of three structures: 1) the outer contour of the humerus; 2) the coracoid process; and 3) the acromion. The CT was taken with patient's arms raised above her head, hence the humeral shaft extended cranially from its head. The caudal slice used in the present study was the most caudal one on which the humeral head was still visible. The cranial slice used was the most cranial one on which both the acromion and the coracoid process were visible. To include the adjoining soft tissue structures a 0.5 cm margin was then added. This put the acromioclavicular joint within the delineated volume.
Arm/shoulder morbidity and clinical assessment
At the time of clinical assessment, patients completed the Kwan's Arm Problem Scale (KAPS) [Citation6,Citation11]. The KAPS is a breast cancer-specific scale developed for the assessment of arm/shoulder morbidity. The KAPS rates the self-reported endpoints pain, stiffness, swelling, use of arm and numbness. The KAPS is rated on a five-point Lickert scale with 1 = no symptom, 2 = little, 3 = some, 4 = substantial and 5 = severe symptom or unable to perform [Citation6,Citation11]. The KAPS has shown good psychometric properties with high reliability and acceptable convergent and discriminant validity [Citation11].
During out-patient follow-up, two physiotherapists experienced in the post-treatment care of breast cancer patients assessed the flexion and abduction of both arms using a goniometer (Supplementary Figures 1 and 2, to be found online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2014.880512). Based on these measurements, and in agreement with a different study [Citation11], the cut-off for the difference in range of motion between the arms was set at 25°, with ≥ 25° considered impaired mobility.
Fibrosis was subjectively assessed by both an experienced oncologist and a physiotherapist. The degree of adherence to the subcutaneous tissue and elasticity/stiffness of the soft tissue was evaluated by inspecting and palpating the tissue in the chest wall and axilla. The presence of tissue fibrosis in the irradiated regions were scored as “0 = none, 1 = little, 2 = some, 3 = substantial and 4 = severe. The two parallel assessments showed high correlation (r = 0.79).
BCRL was assessed using volumetric calculations based on arm circumference at six anatomic landmarks (Supplementary Figure 3, to be found online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2014.880512) [Citation17]. This method has shown high correlation with water displacement volumetry and a high degree of reliability has been demonstrated [Citation17]. Clinically defined BCRL (0 = No, 1 = Yes) comprised a difference of ≥ 10% in volume between the two arms [Citation6,Citation18].
In the overall assessment of patients with arm/shoulder morbidity, we defined the presence of fibrosis > 0, BCRL, or a KAPS score > 1 as an endpoint. No/mild symptoms were typically reflected by a KAPS score of < 3, fibrosis < 2 and no BRCL, while moderate/severe symptoms were indicated by a KAPS score ≥ 3, fibrosis by a score ≥ 2 and impaired mobility by a difference of ≥ 25° between the arms.
Dose-volume histograms and statistical analysis
To investigate a possible correlation between observed arm/shoulder morbidity and RT dose level, DVHs for the shoulder joint were calculated based on the original CT-based RT plan with the delineated shoulder contours. Mean and maximum arm/shoulder doses for each patient were calculated. The relationship between arm/shoulder RT dose levels and clinical endpoints were assessed using Spearman's correlation, with 95% confidence intervals estimated by bootstrapping. Correlation analyses between each patient's DVH and the presence of moderate/severe symptoms were also performed. V5 to V45 in steps of 2.5 Gy were extracted for each patient and used in these correlation analyses. Differences with p-values less than 0.05 were considered statistically significant. Furthermore, multivariate logistic regression analysis with clinical endpoints as binary variables (categorized as no/mild versus moderate/severe) was also performed. Covariates included DVH parameters, surgery (mastectomy/lumpectomy), age, observation time (months/years since treatment) and the number of removed axillary lymph nodes.
Results
After a median observation time of 42 months (29–58), 167 of the included 183 patients (91%) had some arm/shoulder morbidity, whereas only 16 women had none. A relatively high proportion of patients reported moderate/severe arm pain (22% had ≥ grade 3); 43% reported moderate/severe symptoms related to the use of the arm; and 32% had a difference in abduction of ≥ 25° ().
Table II. Arm/shoulder morbidity with different endpoints and morbidity degrees. The number of patients with a specific degree of morbidity for an endpoint is shown.
The cohort-based median of the individual mean and maximum arm/shoulder RT doses were 21.5 and 31.2 Gy, respectively. There was considerable inter-patient variability in these dose parameters, with corresponding ranges of 3.1–47.6 and 5.9–55.4 Gy, respectively. The cohort-based DVHs for the shoulder demonstrated that, on average, large shoulder volumes (typically 50–90% of the volume delineated by CT) received doses between 5 Gy and 20 Gy, whereas smaller shoulder volumes (typically 20% or less) were exposed to doses of 25 Gy or higher (data not shown).
shows correlations between clinical endpoints and RT dose-related parameters. The shoulder volumes receiving approximately 15 Gy were also included. The clinical endpoints assessed by the KAPS, such as pain, stiffness, swelling, use of arm and numbness were significantly associated with each other (p < 0.05). For the RT-related parameters, shoulder volumes receiving approximately 15 Gy were significantly associated with more than one of the clinical endpoints. shows the correlation between arm/shoulder volumes receiving a dose of approximately 15 Gy and the KAPS score for the use of arm. The larger the arm/shoulder volume receiving approximately 15 Gy, the higher the KAPS score. When abduction difference was considered a binary variable (≥ 25° vs. < 25°), a significant correlation between abduction difference of ≥ 25° and shoulder volumes receiving approximately 15 Gy was found (r = 0.18, p = 0.017) (data not shown).
Figure 2. Correlation between dose and the shoulder volume (%) receiving 15 Gy or more (V15) with Use of arm as endpoint assessed by KAPS scores between 1 and 5 (1 = no symptom and 5 = severe symptom). Median values are indicated as horizontal bars.
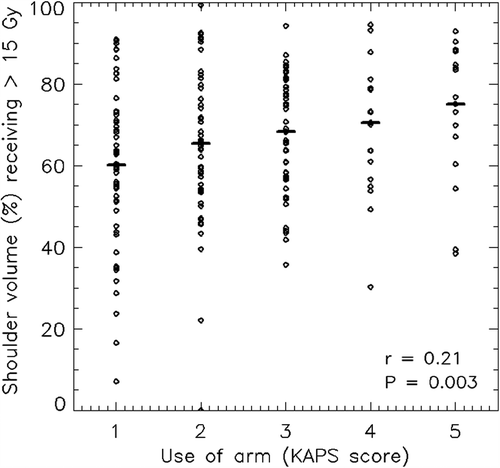
Table III. Spearman's correlation between clinical endpoints and arm/shoulder dose. Statistically significant correlations are indicated with a cross.
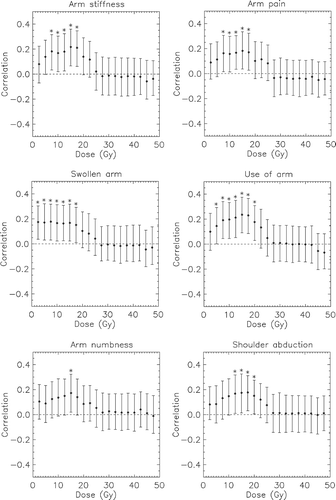
In , only clinical endpoints that had a significant association with DVH parameters were included. Significant correlations between DVHs and arm pain, arm stiffness, swollen arm, use of arm, arm numbness and an abduction difference of ≥ 25° were found, typically for shoulder volumes receiving doses between 5 Gy and 20 Gy. Regardless of the endpoint, the highest correlation was often found at doses of approximately 15 Gy. No significant correlations between DVH parameters and the other clinical endpoints (shoulder flexion, fibrosis and BCRL) were found.
Figure 3. Spearman's correlation between clinical endpoints and the shoulder volume receiving more than the specified dose, with 95% confidence interval indicated by vertical error bars. Only endpoints showing any significant correlations with dose are depicted. (*: significant correlation).
In multivariate logistic regression analyses, shoulder volumes receiving approximately 15 Gy were included as the most relevant DVH parameter. The only other covariate that was significantly correlated with clinical endpoints was surgery, which showed associations with fibrosis and BCRL (p-values of 0.049 and 0.041, respectively). In addition, surgery was significantly associated with arm stiffness and use of arm (p-values of 0.014 and 0.012, respectively). Including surgery as a covariate reduced the predictive impact of shoulder volumes receiving approximately 15 Gy for arm swelling and arm pain only, but it remained significantly associated with these endpoints (data not shown).
Discussion
Among the different clinical endpoints considered, arm pain, use of arm and shoulder abduction dominated in the majority of our patients. Shoulder volumes receiving approximately 15 Gy was shown to be significantly correlated with arm pain, arm stiffness, swollen arm, use of arm, numbness and shoulder abduction. However, the associations between the RT-related parameters and each clinical endpoint were not strong, indicating that the observed late effects are caused by multiple treatment factors related to surgery, chemotherapy and RT.
RT is a part of the standard treatment for breast cancer patients receiving conservative surgery, and for those at risk of local recurrence after mastectomy. Despite the use of less extensive surgery, and whenever possible the reduction of RT to the axilla, arm/shoulder morbidity is still observed. Armer et al. [Citation19] reported numbness, tenderness and pain as the most prevalent symptoms among their patients. In a recent study by Lundstedt et al. [Citation20] paraesthesia was reported as a symptom after RT to the supraclavicular lymph nodes in breast cancer patients. The START trials [Citation8] showed arm/shoulder pain in up to a third of the patients over five years after treatment, and around 20% of the patients complained about shoulder stiffness. A substantial proportion of women (91%) included in the current study reported mild to severe arm/ shoulder symptoms, which is consistent with the studies mentioned above.
Blomqvist et al. [Citation21] reported that patients receiving axillary RT (as opposed to chest wall RT alone) are at higher risk for late arm/shoulder morbidity. In our study, using the four-field photon technique including axillary RT, significant correlations between several endpoints and shoulder volumes receiving approximately 15 Gy were found.
In a review by Violet et al. [Citation22] both the incidence and severity of BCRL were found to be related to the extent of axillary surgery and the type of breast surgery, and the overall effect was markedly increased by the addition of axillary RT. Nesvold et al. [Citation23] reported an increasing prevalence of BCRL as a result of modified radical mastectomy, increasing number of metastatic lymph nodes, age and overweight. In the present study, no significant correlation between arm/shoulder RT dose level and the prevalence of BCRL was observed, whereas the surgical procedure (mastectomy/lumpectomy) in the multivariate analyses correlated significantly (p < 0.05) with BCRL. Therefore, we cannot claim that patients treated with RT are more prone to BCRL, as was reported in some earlier studies. Adriaenssens et al. [Citation24] indicated that weight gain and axillary lymph node dissection contributed to an increase in arm volume for both the ipsilateral and contralateral limb. It was unclear for the authors [Citation24] why axillary dissection on one side should affect the contralateral limb, but chemotherapy was mentioned as a contributing factor. In the present study, no increased in BCRL was observed among patients with a high number of lymph nodes removed (data not shown). This could be a result of the fact that patients with a high number of axillary lymph nodes removed received lower RT doses to the axilla. Our results are in agreement with the results of a recent study carried out by Rutgers et al., who reported significantly less lymphedema in patients treated with RT than with surgery [Citation25]. Bentzen et al. [Citation26] concluded that in the case of lymphedema, patients’ age and obesity are the only risk factors that have been established with some certainty. Nesvold et al. [Citation23] reported a strong relationship between modified radical mastectomy, body mass index and BCRL. Taking these observations together, it seems that the major cause for BCRL development is surgery, not RT.
Muscles close to the shoulder may have an impact on post-treatment arm/shoulder morbidity if they are in the field of surgery/RT. Shamely et al. [Citation27] found that the pectoralis major and minor muscles decreased in size on the affected side in a series of 57 breast cancer patients from six months to six years post-surgery. These authors [Citation27] concluded that patients treated for breast cancer had altered muscle activity in three key muscles that act on the shoulder (upper trapezius, pectoralis major and rhomboid), despite of the fact that two of the key muscles (upper trapezius and rhomboid) were not in the field of surgery/RT. In our study the delineation of the shoulder joint included the bony humeroscapular joint linked to muscles, tendons, ligaments and adjoining soft tissue structures. In this way the dose to the whole area of importance concerning arm/shoulder morbidity, as discussed in these studies, was assessed. Bentzen et al. [Citation26] hypothesized that damage inflicted by RT on the pectoralis major muscle was a significant factor in restricted shoulder mobility even without clinically detectable subcutaneous tissue fibrosis. Ludwig et al. [Citation28] suggested that fibrosis in the pectoralis major and minor muscles could be a factor in restricted shoulder mobility. Our study's low correlation between RT dose level and fibrosis might be explained by the limited number of patients suffering from moderate/severe fibrosis (9%), although a significant correlation between surgery and fibrosis was found.
Some methodological aspects concerning the clinical assessment of shoulder mobility using goniometer should be kept in mind. One study, using ≥ 15° difference between the two arms as definition of restricted shoulder mobility, found no significant correlation with the subjective assessment of mobility in a sample of breast cancer patients (N = 110) [Citation29]. We concluded that a difference of ≥ 25° between the two shoulders was more representative for the treatment-related mobility restriction, which is comparable with other studies [Citation30]. A limitation of this assessment is that minor shoulder problems with other etiologies, which can also result in restricted mobility, might have been overlooked, even though we intentionally excluded women with obvious shoulder impairments unrelated to breast cancer from our analyses.
We acknowledge several limitations in the present study. Ten years have elapsed since our survey, warranting a truly long-term survey related to clinical endpoints. However, we believe the presented correlations between dose volume and clinical endpoints a median 3.5 years after treatment merit their own publication, especially since the same RT technique is still is used at our institution. The effect of body weight on BCRL was not examined in this study. The lack of pre-treatment assessment of shoulder function is another limitation, as surgery was done at hospitals in different regions of Norway. The effect of chemotherapy and hormone treatment on BCRL was not examined in this study, and the assessment of fibrosis was based only on the observers’ subjective evaluation. In this explorative study a p-value of < 0.05 was regarded as significant despite multiple testing, which may have resulted in false-positive associations. Still, shoulder volumes receiving approximately 15 Gy were found was significantly correlated with many different endpoints.
In conclusion, in this cohort-based study significant correlations were found between shoulder joint volume receiving a dose of approximately 15 Gy and clinical endpoints such as arm pain, arm stiffness, swollen arm, use of arm, numbness and impaired shoulder abduction. Post-treatment arm/shoulder morbidity may be more dependent on the shoulder volume receiving a specific dose than on the dose itself.
Supplementary Figures 1–3
Download PDF (803.9 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol 2011;50: 187–93.
- Early Breast Cancer Trialists’ Collaborative Group. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707–16.
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;366:2087–106.
- Steele GD Jr, Jessup LM, Winchester DP, Murphy GP, Menck HR. Clinical highlights from the National Cancer Data Base: 1995. CA Cancer J Clin 1995;45:102–11.
- Vallis KA, Tannock IF. Postoperative radiotherapy for breast cancer: Growing evidence for an impact on survival. J Natl Cancer Inst 2004;96:88–9.
- Nesvold IL, Fosså SD, Holm I, Naume B, Dahl AA. Arm/shoulder problems in breast cancer survivors are associated with reduced health and poorer physical quality of life. Acta Oncol 2010;49:347–53.
- Peuckmann V, Ekholm O, Rasmussen NK, Groenvold M, Christiansen P, Møller S, et al. Chronic pain and other sequelae in long-term breast cancer survivors: Nationwide survey in Denmark. Eur J Pain 2009;13:478–85.
- Hopwood R, Haviland JS, Sumo G, Mills J, Bliss JM, Yarnold JR, et al.; START trial management group. Comparison of patient-reported breast, arm and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow-up in the randomised Standardisation of Breast Radiotherapy (START) trials. Lancet Oncol 2010;11:231–40.
- Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Hölzel D. Axillary surgery severely affects quality of life: Results of a 5-year prospective study in breast cancer patients. Breast Cancer Res Treat 2003;79:47–57.
- Quinlan E, Thomas-MacLean Roanne, Hack T, Kwan W, Miedema B, Tatemichi S, et al. The impact of breast cancer among Canadian women: Disability and productivity. Work 2009;34:285–96.
- Nesvold IL, Fosså SD. Kwan's arm problem scale: Psychometric examination in a sample of stage II breast cancer survivors. Breast Cancer Res Treat 2009;117:281–8.
- Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001;92:1368–77.
- Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: A meta- analysis of treatment factors. Ann Surg Oncol 2009;16: 1959–72.
- Johansen J, Overgaard J, Blichert Toft M, Overgaard M. Treatment morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol 2000;39: 349–54.
- Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 2009;302: 1985–92.
- Bentzen SM, Overgaard M, Thames HD. Fractionation sensitivity of a functional endpoint: Impaired shoulder movement after post mastectomy radiotherapy. Int J Radiat Biol Phys 1989;17:531–7.
- Karges JR, Mark BE, Stikeleather SJ, Worrell TW. Concurrent validity of upper extremity volume estimates: Comparison of calculated volume derived from girth measurements and water displacement volume. Phys Ther 2003;83:134–45.
- Kwan W, Jackson J, Weir LM, Dingee C, McGregor G, Olivotto IA. Chronic arm morbidity after curative breast cancer treatment: Prevalence and impact on quality of life. J Clin Oncol 2002;20:4242–8.
- Armer J, Fu MR. Age differences in post-breast cancer lymphedema signs and symptoms. Cancer Nurs 2005;28: 200–7.
- Lundstedt D, Gustafsson M, Steineck G, Alsadius D, Sundberg A, Wilderäng U, et al. Long-term symptoms after radiotherapy of supraclavicular lymph nodes in breast cancer patients. Radiother Oncol 2012;103: 155–60.
- Blomqvist L, Stark B, Engler N, Malm M. Evaluation of arm and shoulder mobility and strength after modified radical mastectomy and radiotherapy. Acta Oncol 2004;43: 280–3.
- Violet JA, Charmer C. Breast cancer: Improving outcome following adjuvant radiotherapy. Br J Radiol 2004;77: 811–20.
- Nesvold IL, Dahl AA, Løkkevik E, Marit Mengshoel A, Fosså SD. Arm and shoulder morbidity in breast cancer patients after breast-conserving therapy versus mastectomy. Acta Oncol 2008;47:835–42.
- Adiaenssens N, Vinh-Hung V, Miedema G, Versmessen H, Lamote J, Vanhoeij M, et al. Early contralateral shoulder-arm morbidity in breast cancer patients enrolled in a randomized trial of post- surgery radiation therapy. Breast Cancer (Auckl) 2012;6: 79–93.
- Rutgers EJ, Donker M, Straver ME, Meijnen P, Cornelis JH, Velde VD, et al. Radiotherapy or surgery of the axilla after a positive node in breast cancer patients: Final analysis of the EORTC AMAROS trial (10981/220223). 2013 ASCO University Annual Meeting; Available from: http://meetinglibrary.asco.org/content/109779–132.
- Bentzen SM, Overgaard M, Thames HD. Fractionation sensitivity of a functional endpoint: Impaired shoulder movement after post-mastectomy radiotherapy. Int J Radiat Oncol Biol Phys 1989;17:531–7.
- Shamely DR, Srinanaganathan R, Weatherall R, Oskrochi R, Watson M, Ostlere S, et al. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res Treat 2007;106:19–27.
- Ludwig PM, Cook TM, Nawocszenski DA. Three dimensional scapula orientation and muscle activity at selected positions of humeral elevation. JOSPT 1996;24: 47–65.
- Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol 2000;39:393–7.
- Levangie PK, Drouin J. Magnitude of late effects of breast cancer treatment on shoulder function: A systematic review. Breast Cancer Res Treat 2009;116:1–15.

