Abstract
Background. Eosinophilia may represent an early paraclinical sign of malignant disease and a host anti-tumor effect. The association between eosinophilia and the development of solid tumors has never before been examined in an epidemiological setting. The aim of the present study was to investigate eosinophilia in routine blood samples as a potential biomarker of solid tumor development in a prospective design.
Material and methods. From the Copenhagen Primary Care Differential Count (CopDiff) Database, we identified 356 196 individuals with at least one differential cell count (DIFF) encompassing the eosinophil count during 2000–2007. From these, one DIFF was randomly chosen and categorized according to no (< 0.5 × 109/l), mild (≥ 0.5–1.0 × 109/l) or severe (≥ 1.0 × 109/l) eosinophilia. From the Danish Civil Registration System and the Danish Cancer Registry we ascertained all-cause death and solid tumors within the first three years following the DIFF. Using multivariable logistic regression, odds ratios (OR) were calculated and adjusted for previous eosinophilia, sex, age, year, month, C-reactive protein, previous cancer and Charlson's Comorbidity Index.
Results. The risk of bladder cancer was increased with mild eosinophilia [OR 1.93 (CI 1.29–2.89), p = 0.0013]. No associations with eosinophilia were observed for the remaining solid cancers.
Conclusion. We demonstrate that eosinophilia in routine blood samples associates with an increased risk of bladder cancer. Our data emphasize that additional preclinical studies are needed in order to shed further light on the role of eosinophils in carcinogenesis, where it is still unknown whether the cells contribute to tumor immune surveillance or neoplastic evolution.
In healthy individuals, eosinophilic granulocytes (eosinophils) constitute less than 5% of the total white blood cells [Citation1]. However, in some diseases the number of eosinophils may increase, sometimes markedly. Various stimuli may activate eosinophils making them capable of secreting proteins with pivotal function in infection, allergy and inflammation, including inflammatory processes accompanying some solid and hematological malignancies [Citation2,Citation3]. Eosinophilia (> 0.5 × 109/l peripheral blood) is in these cases termed reactive or secondary. Hematological cancers may also sometimes themselves “drive” the eosinophilia as part of an autonomous condition in which case the eosinophilia is termed primary or clonal. Lastly, the term idiopathic hypereosinophilic syndrome is used to classify patients with eosinophilia that does not seem to derive from primary or secondary genesis [Citation3,Citation4]. Irrespective of the cause of the activation, however, the eosinophils may have diverse physiological functions and cause organ involvement [Citation3,Citation4].
Recently, eosinophilic cationic protein was shown to exert a cytotoxic effect on Hodgkin's lymphoma (HL) cell lines, which represent a malignant hematologic disease where concomitant eosinophilia is prevalent in about 15% of cases at diagnosis [Citation5]. Therefore, blood eosinophilia preceding the diagnosis of HL may represent an immunological defense mechanism against the neoplastic cells. Similarly, tissue infiltration by eosinophils has been associated with a favorable prognosis in various solid tumors [Citation6], and evidence of an immunological anti-tumor mechanism in prostate cancer has also been presented [Citation7]. In keeping with these findings, eosinophils have most recently been shown to express various pattern recognition receptors (PRRs) which are implicated in immune functions much more diverse than known from mere microbial protection [Citation8].
Eosinophils can interact with other cell types and may hereby play a role in modulating immune responses. In solid tumors tissue destruction caused by neoplastic cell growth generate so-called danger-associated molecular patterns which bind to PRRs; also on eosinophils [Citation8]. The exact role of the eosinophils in the tumor microenvironment is still unclear, however. It is unknown whether they exert a toxic effect on neoplastic cells through exocytosis of granules or an opposite tumor promoting effect through inappropriate immunoregulatory functions [Citation8]. Accordingly, blood and tumor tissue eosinophilia has been linked to both favorable and unfavorable responses in different solid cancers [Citation6].
Hence, eosinophilia may in some cases represent an early paraclinical sign of malignant disease and perhaps a host anti-tumor effect. The role of the eosinophil in the tumor microenvironment has received increasing attention over the last decade, but the association between eosinophilia and the development of solid tumors has never before been examined in an epidemiological setting. The aim of the present study was to investigate eosinophilia in routine blood samples as a potential biomarker of solid tumor development in a prospective design.
Material and methods
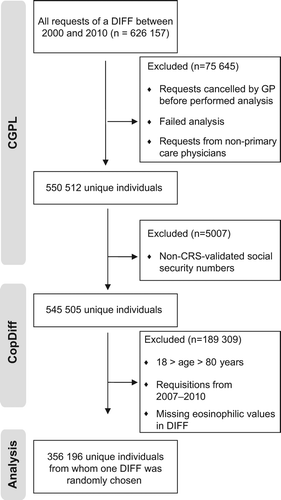
The Copenhagen General Practitioners’ Laboratory (CGPL) is the laboratory for all general practitioners in the Copenhagen area covering approximately 1.1 million inhabitants. CGPL has International Organization for Standardization (ISO) accreditation and has registered all analytical results since 1 May 2000. The Copenhagen Primary Care Differential Count (CopDiff) Database contains results from all differential cell counts (DIFF) requested by general practitioners in Copenhagen from 1 May 2000 to 25 January 2010. From each of the 359 950 unique individuals (aged 18–80 years) with at least one DIFF in the period 1 January 2001 to 31 December 2007, one DIFF encompassing the eosinophil count was randomly chosen by computer-generated random numbers (n = 356 196) (). The individuals were categorized according to no (< 0.5 × 109/l), mild (≥ 0.5–1.0 × 109/l) or severe eosinophilia (≥ 1.0 × 109/l). Where available the level of C-reactive-protein (CRP), categorized as “increased” (≥ 10 mg/l) versus “normal” (< 10 mg/l) was also obtained from the database. Furthermore, we recorded whether another DIFF was made during six months before the request and whether eosinophilia was present in this DIFF. In April 2011, the CopDiff database was linked to: 1) The Danish Civil Registration System (CRS) listing everyone living in Denmark with a permanent and unique personal identification number, which gives information on vital status and enables linkage between study populations and all national registries; 2) The Danish Cancer Registry (DCR), containing data on all malignancies in Denmark since 1942 and to which reporting is mandatory [Citation9]; and 3) The Danish National Patient Register (NPR) including information on all contacts with hospitals in Denmark, inclusive of discharge diagnoses and surgical procedures performed [Citation10]. To adjust for possible confounding by comorbid conditions, we computed Charlson's Comorbidity Index (CCI) [Citation11] from the hospital contacts recorded in the NPR within three years before the index DIFF.
Figure 1. Flowchart. CGPL, Copenhagen General Practitioners’ Laboratory; CopDiff, Copenhagen Primary Care Differential Count Database; CRS, The Danish Civil Registration System; DIFF, differential cell count; GP, general practitioner.
Outcomes were three-year all-cause mortality (taken from the CRS) and incidences of solid tumors as defined by the International Classification of Diseases (ICD) version 10 over the three-year period following the DIFF: buccal cavity and pharynx (C00-C14; C462), digestive organs (C15-C26), respiratory system including thoracic organs (C30-39; C450), bones, joints and articular cartilage (C40-C41), skin (C43-C44; C460), mesothelium and connective tissue (C451-C459; C461; C463; C467; C468 C469; C47-C49; B210), breast (C50), female genital organs (C51-C58), male genital organs (C60-C63), urinary tract (C64-C68; D090-D091; D301-D309; D411-D419), eye and central nervous system (C69-C72; C751-C753; D32-D33; D352-D354; D42-D43; D443-D445), and endocrine glands (C73-C74; C750; C754-C759).
Statistical analysis
We used multivariable logistic regression to compute odds ratios (ORs) with 95% confidence intervals (CIs) for the three-year incidence of all-cause death and solid tumors following the index DIFF. The ORs were adjusted for sex, age (quadratic), year, month, CCI, CRP and previous eosinophilia. Individuals with previous cancer (n = 22 250) were omitted from the analyses. To account for multiple statistical testing, p-values less than 0.0022 were regarded to be significant as this controls the false discovery rate at 5% using the method of Benjamini-Hochberg [Citation12]. All analyses and calculations were performed with SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
In the total cohort of 359 950 individuals there was a female/male sex ratio of 1.38 and a mean age of 48.3 years (). In total 14 406 individuals (4%) exhibited eosinophilia. The incidence of solid cancer was 1090 per 100 000 person-years (). Overall, we observed no association between eosinophilia and the subsequent risk of solid cancers with odds ratios for mild and severe eosinophilia of 0.94 (0.85–1.03), p = 0.18 and 0.98 (0.74–1.30), p = 0.90, respectively. The risk for bladder cancer, however, was increased with mild eosinophilia, OR 1.93 (1.29–2.89), p = 0.0013. Also, eosinophilia showed the known association with all-cause death in a dose-dependent fashion.
Table I. Patient characteristics.
Table II. The association between eosinophilia and 3-year incidence of solid tumors (n = 356 196).
Discussion
In oral, gastric and breast cancer a cellular infiltration of eosinophils in tumor tissue has been described [Citation6]. The presence of tissue eosinophilia has also been associated with favorable outcomes for both carcinoma of the cervix, primary lung cancers as well as gastric and colon carcinoma [Citation6]. In this first epidemiological study on the topic, we also present a link between eosinophilia and carcinogenesis by demonstrating that eosinophilia in routine blood samples may function as a biomarker as it is associated with an increased incidence of bladder cancer. It is an interesting hypothesis that the eosinophil may function as a mediator during the evolution of some, but apparently not all, solid tumors. In support of our finding for bladder cancer, tumor-associated tissue eosinophilia has been shown to correlate to a higher mean survival for patients with pure transitional cell tumors [Citation13] and importantly, eosinophilia has been reported to be more common in bladder tumors at a late stage of invasion [Citation13,Citation14]. The latter may explain the observed increased risk for being diagnosed with bladder cancer after an index blood count exhibiting eosinophilia. The in vivo role(s) of the eosinophils in bladder cancer remain(s) unknown and we cannot contribute to this discussion with our data.
However, this study has other important strengths. 1) All malignant diagnoses in this study were derived from the DCR which was established in 1942 and to which reporting is mandatory. Measures to maintain validity and quality are secured through rigorous quality control routines applied in the daily production and in completing annual reports [Citation9]. 2) The use of logistic regression in The CopDiff database on the three-year incidence ensures that the measures of risk (OR) can be interpreted independently of the frequency of the outcomes in the study, and the OR is therefore a valid estimate for risk in the general population as well. Is it important to emphasize, however, that absolute risks of solid tumors among these individuals are low; the majority of cases of eosinophilia will be reactive to benign conditions. 3) We observed an incidence of solid tumors of 1090/100 000 person-years which is compatible with the reported national incidence of 1205/100 000 person-years suggesting that the CopDiff database does not differ much from the general population with respect to cancer incidence [Citation15]. 4) The CopDiff population was sampled continuously without any restrictions as to why the differential count was requested by the general practitioner. This, together with the use of a computer-generated random selection among these DIFFs avoids surveillance bias to a large extent as the patients are not sampled at the time of any diagnosis [Citation16].
This study also has important limitations. 1) Our methods of analysis did not allow for an assessment of duration of blood eosinophilia and therefore individuals who might have had time-limited secondary eosinophilia were not excluded. However, eosinophil-related end-organ damage from both clonal and reactive causes has been reported, and a dose-dependent relation between levels of blood eosinophils and/or duration of eosinophilia, and the subsequent risks of end-organ damage has not been demonstrated. Accordingly, we decided not to exclude such patients. 2) A number of risk factors have been identified for bladder cancer, including smoking, benzene and other chemicals [Citation17], but none of these established risk factors have been shown to induce eosinophilia. Schistosomiasis infections may be related to both bladder cancer and eosinophilia [Citation18], but this infection is extremely rare in Denmark. 3) Severe eosinophilia was not associated with bladder cancer, and we presume that this apparent lack of dose-response relation is caused by individuals who died in the fixed time period after the DIFF. Such individuals will have an artificially low probability of experiencing the outcome; a significant association between eosinophilia and all-cause death supports this notion (). 4) The follow-up period of three years is relatively short. The period was chosen in order to link the eosinophil count to the specified outcomes with a reasonable certainty, which would have been more difficult with longer follow-up. We therefore cannot rule out that some eosinophilia-related cancers appeared after the three-year time window due to the latency period for cancer development. This may contribute to explaining why we did not observe any associations with cancer types where tumor-tissue eosinophilia previously has been reported [Citation6]. Also, we investigated the significance of blood eosinophilia while earlier research focused on the association between tumor tissue eosinophilia and cancer.
In conclusion eosinophilia in routine blood samples does not associate with solid tumors in general, but blood eosinophilia does associate with an increased risk of bladder cancer. These findings add to the results from clinical studies of cancer patients exhibiting eosinophilia. In addition, recently published results from preclinical studies demonstrate eosinophils to have previously unknown diverse physiological functions. Given the increased interest in immunotherapy for cancer patients, further knowledge on the interaction between the different white blood cells is warranted. Our data emphasize that additional studies are needed in order to shed further light on the role of the eosinophil not only as a biomarker of certain malignant disease entities, but also in carcinogenesis, where it is still unknown whether the cell contributes to tumor immune surveillance or neoplastic evolution.
Acknowledgments
Christen Lykkegaard Andersen wishes to thank The Danish Cancer Society, which has granted a three-year scholarship (2010–2013). The authors would also like to express their gratitude to Eva & Henry Frænkels’ memorial foundation and Axel Muusfeldts memorial foundation for financial support and to Willy Karlslund, The Research Unit for General Practice and Section of General Practice, Department of Public Health, University of Copenhagen, Denmark for skillful technical assistance. None of the sponsors have had any role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. Christen Lykkegaard Andersen co-designed the study, collected, analyzed and interpreted data and drafted the manuscript. Volkert Siersma performed the statistical analyses. Hans Carl Hasselbalch, Hanne Lindegaard, Hanne Vestergaard, Volkert Siersma and Peter Felding analyzed and interpreted data. Niels de Fine Olivarius and Ole Weis Bjerrum co-designed the study, collected, analyzed and interpreted data. All authors revised the manuscript critically for important intellectual content, and approved the version to be submitted.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol 2009;101:81–121.
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol 2006;24:147–74.
- Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: Secondary, clonal and idiopathic. Br J Haematol 2006;133: 468–92.
- Valent P, Gleich GJ, Reiter A, Roufosse F, Weller PF, Hellmann A, et al. Pathogenesis and classification of eosinophil disorders: A review of recent developments in the field. Expert Rev Hematol 2012;5:157–76.
- Glimelius I, Rubin J, Fischer M, Molin D, Amini RM, Venge P, et al. Effect of eosinophil cationic protein (ECP) on Hodgkin lymphoma cell lines. Exp Hematol 2011; 39:850–8.
- Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood 1992;79:3101–9.
- Furbert-Harris P, Parish-Gause D, Laniyan I, Hunter KA, Okomo-Awich J, Vaughn TR, et al. Inhibition of prostate cancer cell growth by activated eosinophils. Prostate 2003;57: 165–75.
- Kvarnhammar AM, Cardell LO. Pattern-recognition receptors in human eosinophils. Immunology 2012;136:11–20.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health 2011;39(7 Suppl):42–5.
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39(7 Suppl): 30–3.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis 1987;40: 373–83.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soc B 1995;57:289–300.
- Lowe D, Fletcher CD, Gower RL. Tumour-associated eosinophilia in the bladder. J Clin Pathol 1984;37:500–2.
- Lowe D, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: A review. J Clin Pathol 1981;34:1343–8.
- The Danish Cancer Registry. [cited 2013 Mar 22]. Available from: http://www.sst.dk/publ/Publ2011/DAF/Cancer/Cancerregisteret2010pdf.
- Haut ER, Pronovost PJ. Surveillance bias in outcomes reporting. JAMA 2011;305:2462–3.
- Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology 2005;66(6 Suppl 1):4–34.
- Zaghloul MS, Gouda I. Schistosomiasis and bladder cancer: Similarities and differences from urothelial cancer. Expert Rev Anticancer Ther 2012;12:753–63.

