Abstract
Background. Comorbidity is an important prognostic factor for survival in other cancers, but the importance in soft tissue sarcoma has not yet been clarified. The aims of this study were to examine the prevalence of comorbidity in soft tissue sarcoma patients, and estimate the impact of comorbidity on overall and disease-specific mortality.
Material and methods. Overall, 1210 adult patients with soft tissue sarcoma in the extremities or trunk wall were identified through the Aarhus Sarcoma Registry, a validated population-based database. Information on comorbidity was obtained through the National Patient Registry, and a Charlson's Comorbidity score was calculated for each patient. The prevalence of comorbidity was assessed overall, as well as according to age and year of diagnosis. Overall and disease-specific mortality rates according to level of comorbidity were computed. The prognostic value of comorbidity was estimated using crude and adjusted Cox proportional hazard models.
Results. The overall prevalence of comorbidity was 25%. The prevalence increased with increasing age, and patients with comorbidity had a larger proportion of adverse prognostic factors when compared to patients without comorbidity. The five-year disease-specific mortality was 26% (95% CI 24–29) for patients without comorbidity, compared to 33% (95% CI 24–42), 41% (95% CI 32–50), and 44% (95% CI 33–55) for patients with mild, moderate, and severe comorbidity, respectively. After adjusting for age, sex, stage, tumor size, depth, grade, surgical margin, radiotherapy, and chemotherapy, comorbidity was independently associated with an increased overall and disease-specific mortality.
Conclusion. Patients with comorbidity had significantly increased overall and disease-specific mortality compared to patients without comorbidity, even when adjusting for important prognostic factors including age.
The incidence of soft tissue sarcoma increases with age [Citation1]. A demographic shift in the age distribution of the general population is expected in the future, resulting in more elderly patients, who more often suffer from other chronic diseases, i.e. comorbidity, which may influence the treatment strategy, and thus survival [Citation2]. Comorbidity has proven to be an important prognostic factor for survival in other cancer types, even when adjusting for other significant factors such as age, disease stage, and treatment [Citation3–6].
To our knowledge only two studies have investigated the impact of comorbidity on survival in soft tissue sarcoma [Citation7,Citation8]. These studies, including adult, high-grade, non-metastatic, primary and adult extremity or trunk soft tissue sarcoma, respectively, reported no predictive value of comorbidity when comparing patients with and without comorbidity [Citation7,Citation8]. However, the prevalence and prognostic impact of comorbidity has not yet been investigated in a larger population-based series of soft tissue sarcoma. The Aarhus Sarcoma Registry (ASR), a validated population-based sarcoma registry, provides a unique possibility to examine the impact of comorbidity in a large series of soft tissue sarcoma, while adjusting for other known prognostic factors [Citation1].
The aims of this study were to assess and describe the prevalence of comorbidity in soft tissue sarcoma patients as well as to investigate the impact of comorbidity on overall and disease-specific mortality.
Material and methods
The Danish population is approximately 5.5 million [Citation9]. The healthcare system provides tax-supported, free of charge health care for all residents. Since 1968, all citizens in Denmark have been assigned a unique, 10-digit civil personal registration number (CPR number), allowing for linkage on an individual level throughout all Danish registries [Citation10].
Data sources
All sarcoma patients treated at the Aarhus Sarcoma Centre between 1979 and 2008 are registered in a clinical database, the ASR [Citation1]. The diagnostic program and treatment follow national guidelines, and decisions are made by a multidisciplinary sarcoma team [Citation11,Citation12]. Sarcomas were classified into three grades using the grading system described by Jensen et al. [Citation13]. The primary treatment was surgery aiming for a wide excision, according to the principles of Enneking, followed by radiotherapy for deep-seated intermediate and high-grade sarcomas [Citation14]. The ASR is population-based for western Denmark and includes CPR number, date of diagnosis, as well as detailed validated data on tumor characteristics, treatment, follow-up, and death. Data regarding death includes date of death, cause of death (sarcoma or non-sarcoma), and disease status at time of death (disease-free, localized disease, or metastatic disease).
The National Patient Registry (NPR) contains information on all patients admitted to Danish hospitals since 1977, including outpatient visits since 1995 [Citation15–17]. The registry covers more than 99% of Danish hospital admissions in the period [Citation17]. Data registered in the NPR includes CPR number, date of admission and discharge, as well as discharge diagnoses according to the eighth (before 1994) and 10th version of the International Classification of Disease (ICD-8 and ICD-10). The discharge diagnoses include both main and secondary diagnoses from admissions as well as emergency and outpatient visits.
The Danish Civil Registration system was established in 1968 and contains current and historical information on all persons living in Denmark. Registered data includes CPR number, date of birth, residence, vital status (dead/alive), and date of death. The vital status is registered continuously and is updated on a daily basis [Citation10].
The Danish Cause of Death Registry was initiated in 1875 as a result of the mandatory, by law, completion of death certificates for any death occurring in Denmark. The registry is based on the medical information from the death certificates and contains data on the immediate and underlying cause of death according to the ICD-8 and ICD-10 [Citation18].
Patients
Between 1979 and 2008, 1753 consecutive patients were treated for soft tissue sarcoma at the Sarcoma Centre of Aarhus University Hospital, Denmark. Due to different biological behaviors, we excluded patients younger than 15 years, tumors not located in the extremities or trunk wall, and specific subtypes including gastrointestinal stromal tumors (GIST), kaposis sarcoma, atypical fibroxanthoma, and subcutaneous low-grade liposarcoma, i.e. atypical lipomatous tumor. The study population comprised 1210 adult patients with a soft tissue sarcoma in the extremity or trunk wall.
Comorbidity
The level of comorbidity at the time of sarcoma diagnosis was assessed using the Charlson's Comorbidity Index [Citation19]. The Charlson's Comorbidity Index was originally developed in 1984 to predict one-year mortality in a cohort of 559 medical patients, and was later validated for 10-year mortality in 685 breast cancer patients. The index includes 19 medical conditions, which are weighted from 1 to 6 points according to their risk of mortality. These points are added to form a final score corresponding to the level of comorbidity [Citation19]. The Charlson's Comorbidity Index has been adapted and validated for ICD-based hospital discharge data in various cancer types [Citation20]. The ICD-codes included in the index are shown in Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.888494.
Data from the ASR and NPR were linked through the CPR number, and for each of the 1210 soft tissue sarcoma patients identified in the ASR, all discharge diagnoses registered in the NPR, between 1 January 1977 and the date of the sarcoma diagnosis, were extracted. Based on these discharge diagnoses, a Charlson's Comorbidity Index score for each patient was computed. To eliminate non-specific symptoms or hospital admissions related to the sarcoma, all discharge diagnoses within 30 days, and all cancer diagnoses within 90 days prior to the sarcoma diagnosis were excluded. The level of comorbidity was categorized into four groups; no (score 0), mild (score 1), moderate (score 2), and severe (score ≥ 3) comorbidity.
Mortality
Mortality was assessed as overall and disease-specific mortality. Data on death of the study population was obtained through the Danish Civil Registration system [Citation10]. Patients were followed from the date of sarcoma diagnosis until death, emigration, or end of the study period (15 April 2013), whichever came first. Data on the cause of death was obtained primarily from the ASR and secondarily from the Danish Cause of Death Registry. Disease-specific mortality was defined as death from sarcoma or death with metastatic sarcoma, corresponding to ICD-8; 170, 171, 192.49-99 and ICD-10; C40-C41, C47, C49 in the Danish Cause of Death Registry [Citation18].
Statistical analyses
The prevalence of comorbidity was assessed as an overall proportion, as well as according to age, sex, and calendar year of diagnosis. In order to have comparable data, the analysis of prevalence according to calendar year was confined to patients diagnosed between 1982 and 2008 including comorbidity only five years prior to the sarcoma diagnosis, excluding diagnoses from outpatient visits. The impact of comorbidity on overall and disease-specific mortality was assessed as proportion with 95% confidence intervals (CI) and presented as cumulative incidence functions. The prognostic value of comorbidity on overall and disease-specific mortality was assessed crude as well as adjusted for known prognostic factors; age (years), sex, stage at diagnosis (localized or metastatic), tumor size (cm), depth (subcutaneous or subfascial), grade (low, intermediate or high), surgical margin (intralesional/marginal or wide/radical), radiotherapy (yes or no), and chemotherapy (yes or no). The prognostic factors included in the adjusted analyses were selected a priori, based on a literature review. Age and tumor size were included in the analyses as four-knotted restricted cubic splines [Citation21]. The Cox proportional hazard model was used and results presented as hazard ratios with 95% CI. The proportional hazard assumption was tested using log-minus-log plots. Subgroup analyses were performed according to age, sex, and calendar year of diagnosis. Homogeneity within subgroups was tested using the likelihood ratio test. Death from other causes than sarcoma was considered a competing event in the analyses of disease-specific mortality. All tests were two-sided and a p-value ≤ 0.05 was considered significant. Analyses were performed using Stata, version 11.2.
Ethics
This study was approved by the Danish Data Protection Agency (2007-58-0010) and the Danish Health and Medicines Authority (7-604-04-2/262/KWH), and was conducted in accordance with the Helsinki Declaration.
Results
Patient characteristics and prevalence of comorbidity
Overall, 1210 adult patients were diagnosed with a trunk or extremity soft tissue sarcoma in western Denmark from 1979 to 2008. The median age at diagnosis was 59 years (range 15–95) and 52% were males. At diagnosis, 88% presented with a localized primary tumor. The patient characteristics are shown in . The median follow-up in patients alive at the end of follow-up was 13.1 years, ranging from 2.8 to 34.2 years.
Table I. Patient characteristics by Charlson’s Comorbidity score (N = 1210).
Comorbidity was present in 299 patients, corresponding to a prevalence of 25%. Of these, 106 (35%) patients had mild comorbidity, while 107 (36%) and 86 (29%) patients had moderate and severe comorbidity, respectively. The differences in patient characteristics, according to the Charlson's Comorbidity Index score, are shown in . Generally, the presence of comorbidity was significantly associated with increased age, a larger proportion of metastatic disease at presentation, diagnosis in 1999–2008, grade 3 tumors, intralesional/marginal excision, as well as lack of treatment with radio- and chemotherapy. The prevalence of the medical conditions included in the Charlson's Comorbidity Index is shown in . The most frequent condition, “Any tumor”, was present in 9.9% of cases. The overall prevalence of comorbidity increased with increasing age, peaking at 88 years (43%), as shown in . The prevalence of mild comorbidity was highest at age 79 (13%), compared to age 95 (31%) and 75 (10%) for moderate and severe comorbidity, respectively. No significant change in the overall prevalence of comorbidity over the study period was observed (). However, some minor changes in the composition were seen, with a decrease in moderate comorbidity and an increase in severe comorbidity, most pronounced in the first part of the study period.
Figure 1. Prevalence of Charlson's Comorbidity score as percentage by age (A) and calendar year of diagnosis (B).
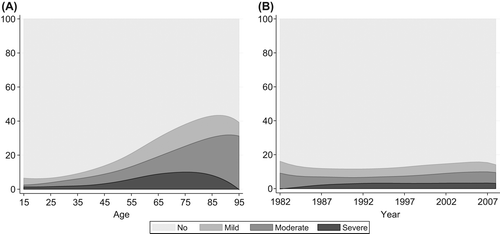
Table II. Overall prevalence of medical conditions in the Charlson’s Comorbidity Index among soft tissue sarcoma patients in the Aarhus Sarcoma Registry (N = 1210).
Overall mortality
The one-, five-, and 10-year overall mortality was 16% (95% CI 14–19), 41% (95% CI 38–44), and 52% (95% CI 49–55), respectively. The overall mortality is significantly affected by the level of comorbidity, as shown in . The five-year overall mortality for patients without comorbidity was 35% (95% CI 32–38), compared to 52% (95% CI 43–62), 62% (95% CI 53–71), and 69% (95% CI 59–78) for patients with mild, moderate, and severe comorbidity, respectively. The crude and adjusted analyses of comorbidity by age, sex, and calendar year at diagnosis are shown in . The impact of comorbidity on overall mortality was significantly different among age groups (p = 0.0028), while not between sex (p = 0.64), nor calendar year of diagnosis (p = 0.97). A tendency towards comorbidity (all levels) being independently prognostic for overall mortality was seen in all age groups and collapsed results were computed. Overall; mild, moderate, and severe comorbidity were independently correlated with an increased overall mortality, compared to no comorbidity ().
Figure 2. Cumulative incidence functions of overall (A) and disease-specific mortality (B) by Charlson's Comorbidity score.
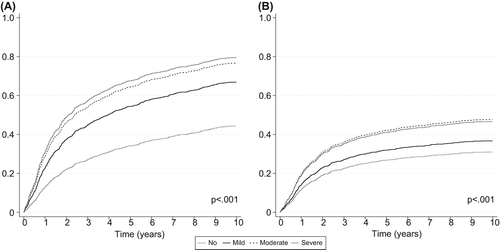
Table III. Crude and adjusted analyses for the effect of comorbidity on overall and disease-specific mortality in 1210 adult soft tissue sarcoma patients by age, sex, and calendar year of diagnosis.
Disease-specific mortality
The one-, five-, and 10-year disease-specific mortality was 13% (95% CI 11–15), 30% (95% CI 27–32), and 34% (95% CI 31–37), respectively. The cumulative incidence function of disease-specific mortality by level of comorbidity is shown in . The five-year disease-specific mortality was 26% (95% CI 24–29) in patients without comorbidity, compared to 33% (95% CI 24–42), 41% (95% CI 32–50), and 44% (95% CI 33–55) in patients with mild, moderate, and severe comorbidity, respectively. The crude and adjusted analyses of comorbidity by age, sex, and calendar year at diagnosis are shown in . The impact of comorbidity among age groups (p = 0.14), sex (p = 0.30), and calendar year of diagnosis (p = 0.94) was not significantly different. Overall; mild, moderate, and severe comorbidity were independent prognostics for disease-specific mortality, corresponding to a 46%, 55%, and 204% increase in mortality rate, compared to patients without comorbidity (). Moderate and severe comorbidity were not associated with an additionally increased rate compared to mild comorbidity (p = 0.79 and p = 0.17, respectively).
Discussion
In this population-based study we found an overall prevalence of comorbidity of 25%. Patients with comorbidity had a significantly increased overall and disease-specific mortality compared to patients without comorbidity, even when adjusting for age and other important prognostic factors. A tendency towards comorbidity being associated with an increased mortality was observed within all subgroups. The impact of comorbidity on disease-specific mortality was consistent within all subgroups; however, the impact on overall mortality differed among the age groups.
Methodological considerations
The main strength of our study lies in the structure of the Danish healthcare system, which facilitates population-based studies with large sample sizes, complete follow-up on all patients, and the possibility of linkage, on an individual level, to clinical databases and registries.
The information on comorbidity was based on an administrative registry, the NPR. Due to the prospective registration of data in the NPR, independently of the aim of our study, the potential information bias is considered low. When using administrative registries, misclassifications or coding errors occur to some extent; however, in this case the comorbidity occurred before the sarcoma diagnosis, and any misclassification is therefore expected to be unrelated to the sarcoma, i.e. non-differential. Outpatient data was only registered in NPR after 1995, which means that minor comorbidity not requiring hospital admission would be missed in the first half of the study period.
The quality of diagnostic coding in NPR for the 19 medical conditions in the Charlson's Comorbidity Index has previously been validated. Thygesen et al. [Citation22] reported an overall positive predictive value of 98% for the 19 conditions, using ICD-10 codes in the NPR in 1998–2007. The positive predictive value varied from 82% for diabetes mellitus with end organ damage to 100% for congestive heart failure, peripheral vascular disease, chronic pulmonary disease, mild and moderate/severe liver disease, hemiplegia, moderate/severe renal disease, leukemia, lymphoma, metastatic tumor solid, and AIDS.
The prevalence of some of the milder conditions, such as diabetes, might be underestimated in the NPR. The negative predictive value for ICD coding in the NPR has, to our knowledge, not been investigated; however, severe diseases with a low prevalence in a population tend to have high negative predictive value, while mild diseases with a high prevalence tend to have lower negative predictive values.
The Charlson's Comorbidity Index used to assess comorbidity is widely used, and has previously been validated [Citation3,Citation4,Citation6,Citation7,Citation20,Citation23]. However, the prevalence and prognosis for some of the 19 medical conditions, e.g. the condition “ulcer disease”, has changed radically since the development of the index. Furthermore, some of the conditions in the Charlson's Comorbidity Index include a wide span of diagnoses with varying severity, e.g. mild and severe chronic obstructive pulmonary disease included in the condition “chronic pulmonary disease”, which are weighed the same. Thus, the Charlson's Comorbidity Index might not capture the “true” mortality risk due to the individual disease, but merely the average risk of multimorbidity. Studying comorbidity as indices is often preferred in order to have sufficient statistical power; however, an update of the index in the future is relevant, especially with focus on the correlation between the level of severity and the impact on sarcoma-specific mortality. Other comorbidity indices exist, however none have proven to be superior thus far [Citation5,Citation24,Citation25].
The outcome in this study was assessed as overall as well as disease-specific mortality. Overall mortality includes both deaths due to sarcoma, as well as deaths due to any other reason, whereas disease-specific mortality only includes deaths due to sarcoma. Data on the cause of death in the Registry of Cause of Death is registered by physicians, either the deceased's general practitioner or hospital doctors, and the validity of the registered causes is thus dependent on the physicians’ knowledge of preceding diseases. In patients with a preceding cancer diagnosis, the risk of stating the cancer as cause of death is increased, causing differential misclassification. Data in the ASR has been systematically validated by two experienced researchers using standardized forms [Citation1]. Data for the cause of death was retrieved from the ASR in the majority of the cases, as the information in the ASR is suspected to be more correct than in the Registry of Cause of Death.
Prevalence
The prevalence of comorbidity was 25%. Two previous studies have investigated the prevalence of comorbidity in soft tissue sarcoma [Citation7,Citation26]. Nakamura et al. [Citation7] reported an overall prevalence of 20%, while van Herk-Sukel et al. [Citation26] reported the prevalence of medical conditions separately, e.g. 33% for cardiovascular disease, 10% for respiratory disease, and 6–7% for diabetes, anemia, and depression. A study of 27 506 newly diagnosed cancer patients, including 413 patients with musculoskeletal tumors, reported a 65% overall prevalence of comorbidity; however, this study included medical conditions not included in the Charlson's Comorbidity Index, e.g. alcohol abuse and obesity [Citation2]. In other cancer types the prevalence of comorbidity has been reported to range from 28% to 43%, when assessed by the Charlson's Comorbidity Index [Citation3,Citation4,Citation6,Citation23]. This difference might be explained by the different etiologies in the different cancer types, e.g. alcohol and smoking in head and neck cancer, which themselves are associated with comorbidity. Furthermore, since the prevalence of comorbidity increases with age, differences in age at diagnosis between different cancer types may affect the prevalence of comorbidity. The median age at diagnosis in our study was 59 years, compared to, e.g. 70 years for renal cancer and 72 years for bladder cancer [Citation4,Citation6]. Contrary to with the findings of other studies, the overall prevalence did not increase over time [Citation4,Citation6,Citation23]. This contradiction may be caused by differences in inclusion or prevalence of diagnoses, since these studies included diagnoses from outpatient visits.
Mortality
Comorbidity significantly impacts survival in various other cancer types such as head and neck, renal, colorectal, and ovarian cancer [Citation3,Citation4,Citation6,Citation23]. Only two studies have, to our knowledge, investigated comorbidity and survival in soft tissue sarcoma [Citation7,Citation8]. The impact of comorbidity on disease-specific survival was investigated in 322 high-grade non-metastatic soft tissue sarcoma patients and 345 patients with tumors located in the extremity and trunk, respectively. Gadgeel et al. assessed comorbidity as a binomial categorical variable (score 0 vs. score 1+), whereas Nakamura et al. assessed comorbidity both as a binomial categorical variable and as a continuous linear variable. Comorbidity was not significant in either of the univariate analyses (Gadgeel et al.: HR = 1.4, p = 0.41. Nakamura: 5-year disease- specific survival score 0: 65% vs. score 1–4: 58.9%, p = 0.58, and HR = 1.306 pr. 1 point increase in score, p = 0.12) and was therefore not analyzed multivariately [Citation7,Citation8]. Contrary to this, we found that comorbidity had a significant independent prognostic impact on disease-specific mortality. The difference in our results might be explained by the different categorization of comorbidity, since our crude analysis of disease-specific mortality showed that moderate and severe comorbidity were statistically significant, while mild was not. Or it can be explained simply by differences in exclusion criteria and sample size.
Patients with comorbidity may be diagnosed earlier, due to a closer contact with the healthcare system, or they may experience a delay in the diagnosis. The latter is supported by our finding that significant more patients with moderate or severe comorbidity had metastases at diagnosis compared to patients with no or mild comorbidity. However, as seen in the adjusted analysis, this difference in stage does not entirely explain the difference in mortality. The treatment of soft tissue sarcoma patients differs according to the disease stage and whereas the treatment of localized disease primarily is surgery, with adjuvant radiotherapy, the treatment of metastatic disease often involves combinations of surgery, radiotherapy, and chemotherapy. The presence of comorbidity might affect the treatment strategy in different ways: the estimated perioperative mortality risk is considered too high, patients might not tolerate chemotherapy, or drugs used to treat comorbid diseases might interact with chemotherapeutic drugs. Whereas the overall proportion of patient treated with surgery was equal regardless of the level of comorbidity, the aggressiveness of surgery, i.e. surgical margin as well as treatment with radiotherapy and chemotherapy was significantly different. This indicates that patients with comorbidity might not be treated optimally in regard to their soft tissue sarcoma in some cases, resulting in a higher sarcoma-specific mortality, even when adjusting for the treatment. However, patients with comorbidity might experience more complications than patients without comorbidity, resulting in a higher morbidity and mortality. There is therefore a need for increased attention regarding comorbidity, especially in patients with metastatic soft tissue sarcoma in order to improve and optimize the treatment.
Conclusion
The prevalence of comorbidity in soft tissue sarcoma patients is relatively low. The level of comorbidity impacts both overall and disease-specific mortality, and patients with mild, moderate, and severe comorbidity had a significantly increased disease-specific mortality compared to patients without comorbidity, even when adjusting for important prognostic factors including age. Soft tissue sarcoma might not be treated optimally in patients with comorbidity, and improved knowledge and awareness of comorbid diseases is important in order to prevent complications and improve treatment.
Supplementary Table I
Download PDF (28.8 KB)Acknowledgments
The study was supported by grants from “Frits, Georg & Marie Cecilie Gluds legat”, “Max & Inge Wørzners mindelegat”, “Erland Richard Frederiksen og Hustrus Legat”, the Danish Council for Independent Research | Medical Sciences, and Aarhus University.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Maretty-Nielsen K, Aggerholm-Pedersen N, Keller J, Safwat A, Baerentzen S, Pedersen A. Population-based Aarhus Sarcoma Registry: Validity, completeness of registration, and incidence of bone and soft tissue sarcomas in western Denmark. Clin Epidemiol 2013;5:45–56.
- Piccirillo JF, Vlahiotis A, Barrett LB, Flood KL, Spitznagel EL, Steyerberg EW. The changing prevalence of comorbidity across the age spectrum. Crit Rev Oncol Hematol 2008;67:124–32.
- Boje CR, Dalton SO, Gronborg TK, Primdahl H, Kristensen CA, Andersen E, et al. The impact of comorbidity on outcome in 12 623 Danish head and neck cancer patients: A population based study from the DAHANCA database. Acta Oncol 2013;52:285–93.
- Lund L, Jacobsen J, Norgaard M, McLaughlin JK, Blot WJ, Borre M, et al. The prognostic impact of comorbidities on renal cancer, 1995 to 2006: A Danish population based study. J Urol 2009;182:35–40.
- Piccirillo JF, Costas I. The impact of comorbidity on outcomes. J Otorhinolaryngol Relat Spec 2004;66:180–5.
- Lund L, Jacobsen J, Clark P, Borre M, Norgaard M. Impact of comorbidity on survival of invasive bladder cancer patients, 1996–2007: A Danish population-based cohort study. Urology 2009;75:393–8.
- Nakamura T, Grimer R, Gaston C, Francis M, Charman J, Graunt P, et al. The value of C-reactive protein and comorbidity in predicting survival of patients with high grade soft tissue sarcoma. Eur J Cancer 2013;49:377–85.
- Gadgeel SM, Harlan LC, Zeruto CA, Osswald M, Schwartz AG. Patterns of care in a population-based sample of soft tissue sarcoma patients in the United States. Cancer 2009;115:2744–54.
- Statbank Denmark. StatBank Denmark. [cited 2012 Jun 15]. Available from: http://statbank.dk/statbank5a/default.asp?w = 1280.
- Pedersen CB. The Danish civil registration system. Scand J Public Health 2011;39(7 Suppl):22–5.
- Danish Health and Medicines Authority. Cancer Pathways, soft tissue and bone sarcoma. [cited 2013 May 24]. Available from: http://www.sst.dk/publ/Publ2012/06juni/KraeftPkforl/SarkomerKnogleBloeddele3udg.pdf.
- Co-operative Cancer Departments. Treatment of sarcoma and aggressive benign tumors. [cited 2013 May 24]. Available from: http://www.ambkir.dk/Faglige%20retningslinier/Behandling%20af%20sarkomer.pdf.
- Jensen OM, Hogh J, Ostgaard SE, Nordentoft AM, Sneppen O. Histopathological grading of soft tissue tumours. Prognostic significance in a prospective study of 278 consecutive cases. J Pathol 1991;163:19–24.
- Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980;(153):106–20.
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39(7 Suppl): 30–3.
- Nickelsen TN. Data validity and coverage in the Danish National Health Registry. A literature review. Ugeskr Laeger 2001;164:33–7.
- Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull 1999;46:263–8.
- Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health 2011;39(7 Suppl):26–9.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40:373–83.
- Tetsche MS, Norgaard M, Skriver MV, Andersen ES, Lash TL, Sorensen HT. Accuracy of ovarian cancer ICD-10 diagnosis in a Danish population-based hospital discharge registry. Eur J Gynaecol Oncol 2005;26:266–70.
- Royston P, Sauerbrei W. Multivariable modeling with cubic regression splines: A principled approach. Stata J 2007;7:45–70.
- Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sorensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol 2011; 11:83–8.
- Tetsche MS, Dethlefsen C, Pedersen L, Sorensen HT, Norgaard M. The impact of comorbidity and stage on ovarian cancer mortality: A nationwide Danish cohort study. BMC Cancer 2008;8:31.
- Baldwin LM, Klabunde CN, Green P, Barlow W, Wright G. In search of the perfect comorbidity measure for use with administrative claims data: Does it exist?Med Care 2006; 44:745–53.
- de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. A critical review of available methods. J Clin Epidemiol 2003;56:221–9.
- van Herk-Sukel MP, Shantakumar S, Overbeek LI, van Boven H, Penning-van Beest FJ, Herings RM. Occurrence of comorbidities before and after soft tissue sarcoma diagnosis. Sarcoma 2012;2012:1–7.

