Abstract
Background. Radiotherapy-induced trismus (RTIT) is a debilitating condition without any proven effective treatment. This study investigates the effectiveness of prophylactic training to prevent RTIT during and up to 12 months after completed RT in patients with head and neck cancer (HNC), also investigating the incidence of RTIT.
Methods. Sixty-six consecutive patients from two RT clinics in Sweden were randomised into one of two groups: training with TheraBite® Jaw Motion Rehabilitation System™ or a control group. Maximum interincisal openings (MIO) were recorded at baseline and once a week during treatment, three, six and 12 months after completed RT. Training frequency was recorded by patients in a log book.
Results. There were no significant differences in MIO between the intervention and control groups at any of the measurement points. Patients in both groups maintained their normal variation in MIO at 12 months after completed RT. A small group of patients in the control group had a 17% mean decrease in MIO by week 6 compared to baseline and improved their MIO by using the training programme. There was a significant mean difference in MIO from baseline to week 6 (3 mm, p = 0.018), and month 6 (2.7 mm, p = 0.040), for patients receiving 3D conformal radiotherapy. There was a significant difference in MIO between patients treated with RT and concurrent chemotherapy compared to patients with RT only at 12 months (p = 0.033).
Conclusions. Patients with HNC undergoing high dose RT do not need to be burdened with an intense prophylactic training programme during RT and up to 12 months after completed RT. MIO measurements during RT and up to 12 months after completed RT are recommended to identify a small risk group who are an exception and may need a training programme.
Head and neck cancer (HNC) is newly diagnosed in about 560 000 individuals worldwide and in Sweden approximately 1200 annually [Citation1,Citation2].
HNC cancer is defined as being tumours arising from the mucosal surfaces of the lip, oral cavity, pharynx, larynx or cervical oesophagus. Other sites included are the nose/paranasal sinuses, salivary/thyroid/ parathyroid glands, or melanoma/non-melanoma skin cancers of the head and neck [Citation3].
Radiotherapy (RT) with or without surgery and/or combined with chemotherapy (CT) is a standard treatment for patients with HNC [Citation4]. For those patients receiving RT, with or without combined CT, both acute and late side effects of the treatment are common. Oral mucositis and oral infections, both resulting in pain, are common acute side effects [Citation5,Citation6], while xerostomia and trismus are late side effects of RT [Citation6]. RT to the jaw muscles (masseter, temporalis, and pterygoids) and the temporomandibular joint [Citation7] causes inflammatory changes which constrict the facilitating muscles of mastication and can lead to muscle fibrosis. This process can cause the onset of radiotherapy-induced trismus (RTIT) [Citation8]. Trismus can lead to other complications such as difficulty in eating, swallowing, speech and general oral hygiene [Citation9].
Incidence of RTIT in patients with HNC varies greatly in older studies, between 5% and 45% as reported in a systematic review with a time period from 1966 to 2003. This was largely attributed to a lack of uniform criteria for RTIT [Citation10]. A cut-off point of ≤ 35 mm for mouth-opening was introduced in 2006 and has been used as a standard measurement for RTIT in more recent studies [Citation11]. A systematic review of 12 articles from 1990 to 2008 reported a weighted prevalence for RTIT to be 25% in patients receiving three-dimensional conventional radiotherapy (3D-CRT), 5% for intensity-modulated radiotherapy (IMRT), and 31% in combined CT and 3D-CRT. The authors suggest that the effects of RT are cumulative, developing slowly and that RTIT may begin towards the end of RT or at any time during the subsequent 24 months. This change is not dependent on treatments and the condition may worsen over time, remain the same or improve [Citation12].
There are varying definitions of RTIT with a cut-off point for mouth opening of between 30 and 40 mm [Citation10], although this does not take into account the individual's normal variation of mouth opening capacity. These variations range from 44 mm to 77 mm (mean 59 mm) in 20-year-old men, and 42 to 75 mm (mean 53 mm) in 20-year-old women, while 70-year-old men with residual teeth range between 44 mm and 65 mm (mean 53 mm) and 70-year-old women with residual teeth range between 38 mm and 59 mm (mean 49 mm) [Citation13,Citation14].
Despite the fact that RTIT has been documented in older literature, there is still limited research about this condition. Two systematic reviews from 2004 and 2010, one investigating the impact of cancer treatments on trismus prevalence [Citation10] and the other identifying criteria, risk factors and treatment interventions for trismus [Citation12], both concluded that there is a need for large well-designed randomised studies investigating the prevention and management of RTIT and the effects of therapeutic interventions. The later systematic review [Citation12] suggests that IMRT may be associated with less frequent prevalence of trismus, and that concurrent CT and RT may be associated with a higher prevalence. When assessing management strategies they concluded that the level of evidence was too low to form guidelines. The only exception was training with a device developed specifically for trismus, the TheraBite® Jaw Motion Rehabilitation System™ (TheraBite) that gave low-grade evidence suggesting it might be effective, but that larger prospective trials are needed to confirm this [Citation12].
Hence, international evidence-based guidelines for patients with HNC experiencing RTIT are lacking [Citation12] and Sweden has no national guidelines, but several clinical regional guidelines are used instead. One from the Stockholm-Gotland region from 2007 which is based on clinical experience [Citation15] suggests that RTIT can partly be prevented by active, passive and supportive stretching of the muscles of mastication during RT.
The purpose of this study was to investigate the effectiveness of standardised prophylactic training to prevent RTIT during and up to 12 months after completed RT. The aim was also to prospectively investigate the incidence of RTIT in patients with HNC.
Material and methods
Patients
Between 2009 and 2013, 66 consecutive patients from two different RT clinics in Sweden, were randomised to receive training with TheraBite or standard treatment. Study selection bias was eliminated by blinded randomisation. Patients were included if they had a planned RT dose of at least 15 Gy to any of the jaw muscles or the temporomandibular joint, maximum interincisal opening (MIO) > 35 mm at the commencement of the study, tooth 11 and tooth 41 intact, a willingness to participate, > 18 years, an ability to understand and communicate in Swedish, and expected compliance to a training programme and follow-up. No edentulous patients were included. Oncologists from one clinic and a dose-planner and an oncologist from the other clinic identified all the patients who met the inclusion criteria and asked about willingness to participate.
Study groups
Patients who gave their written informed consent were randomised into one of two groups: 1) Intervention group (n = 33): RT and training with TheraBite preceded by a warm-up programme; and 2) Control group (n = 33): RT without training. If MIO measurement decreased 15% from the patient's baseline measurement, a training programme was offered (as in the Intervention group.)
Radiotherapy and treatments
All 66 patients received external beam RT. Thirty-five patients were treated with 3D-CRT and 31 patients were treated with IMRT. Most of the patients received high doses in the interval 50–69 Gy. All the patients received 2 Gy per fraction, five times a week giving a range of overall RT times between five and seven weeks. Thirty-five patients received CT (cisplatin 50 mg weekly) in addition to RT. Treatment regimens were given in accordance to tumour size, site and stage of disease.
Description of training programme for the intervention group
TheraBite is a portable device utilising repetitive passive motion and stretching of jaw musculature. The stretching is made comfortable as the opening force is spread over many teeth and protected by foam bite pads. TheraBite has a maximum opening range of 45 mm, which was increased to 64 mm by adding foam pads in the edentulous version when this limitation was discussed with the manufacturer. Patients were given written and verbal information about the usage and maintenance of TheraBite before the commencement of RT. Thereafter, nurses responsible for the study regularly met the patients according to their individual needs to encourage training in the intervention group and to evaluate possible difficulties. Patients were instructed to commence a training pass by softening the jaw muscles with a simple exercise. The training programme consists of five stretches performed five times daily, each stretch held for 15 seconds. Training continued for the entire 12-month follow-up period.
Pilot study
During 2008 a pilot study was conducted to test the logistics and methodology of the main study design. Four men with tonsil cancer consented to be included and all aspects of the methodology were tested. The pilot study supported the intervention methodology and raised the need of a log book for patients to document their training frequency, a check list for the operators, and the importance of visual and written communication regarding the training programme. A manual log was developed to insure that both clinics followed the same methodology.
Data collection
Demographic data
Demographic variables, listed in were collected in a written questionnaire, while clinical data was extracted from patient medical records.
Table I. Patient characteristics (n = 66).
Maximum interincisal opening (MIO)
MIO measurements were assessed at baseline and weekly during RT by a hospital specialist dentist or hygienist. Patients were seated in an upright position. The distance between the opposing incisal edges (incisors 11 and 41) was measured using a MIO slide calibrated calliper in millimetres. We chose to use an initial cut-off criterion of > 35 mm for inclusion to the study, proposed by Dijkstra et al. [Citation11], and supported by Scott et al., [Citation16] while adhering to a 15% decrease in patients individual MIO throughout the study as the determinant of trismus. This was based on recommendations from an established researcher in the field (Professor Tomas Magnusson) claiming that the need to focus on the individual's normal variation in MIO is necessary to be able to detect a clinically relevant impairment of MIO. Variations in percent gave us the ability to compare. Masticator muscles from both sides of the jaw work together causing mandibular dysfunction, so if one side of the jaw is affected the other is automatically affected. Additional MIO measurements were performed at three, six and 12 months after completion of RT.
Training frequency
Patients in the intervention group recorded their training frequency in log books, specially developed for the study. A total of four log books per patient, stretching over four time periods enabled the study operators to assess the frequency of training regularly during the whole follow-up period. Every patient had a scheduled appointment with the study operator in the last week of RT to assess the first training period and encourage the patient to continue their training, using the information in the log book and an additional three questions:
Have you trained or done any stretching of the jaw muscles during RT? (Yes or no answer)
If yes, how often?
Describe how you have trained or stretched?
Patients in the control group were informed that the control group did not include instructions for stretching of jaw muscles, but despite this some of them performed regular stretching. At their scheduled appointment with the study operator in the last week of RT they were asked the three above questions.
Sample size calculation and statistical analysis
Randomisation was performed by the Regional Cancer Centre, Linköping, Sweden. The randomisation lists were generated by computer and were only available to the Regional Cancer Centre staff. Patients were randomised in blocks of eight to receive TheraBite or conventional therapy.
Taking into account the loss of patients, we calculated we would need to recruit 60 patients (30 per treatment group), to be able to detect a reduction in MIO from 90% in the control group to 45% in the intervention group, three months after completed RT. The calculations were done with 80% power at 5% significance level.
All data was entered into an Access database, and all statistical analyses were done with SPSS version 20.0. For comparison of different patient characteristics, we used χ2-test, Fischer's exact test and unpaired-samples t-test. Difference in mean of MIO compared to baseline, and between different groups of MIO measurements, was tested using paired-samples t-test and unpaired-samples t-test. Levene's test was performed to assess variances homogeneity. All tests were two-tailed and conducted at 5% significance level.
Ethical approval
This study was performed according to the ethical standards of the Helsinki Declaration and was approved by the Regional Ethical Review Board in Linköping (M107-09).
The study protocol was published on the Clinical Trials.gov website (identification number NCT01354548).
Results
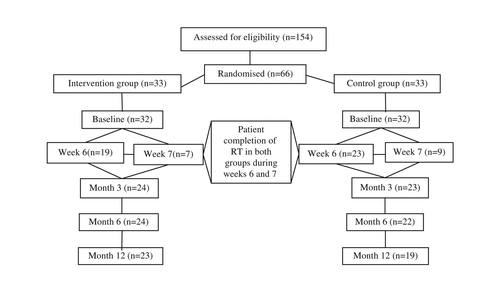
A total of 66 patients were randomised into the study, while 42 patients completed the 12-month follow-up time period (). The 24 drop-outs were evenly distributed between the two groups and were a result of death (n = 5), failure to comply (n = 9), logistical problems (n = 7), or withdrawal (n = 3). There were no significant differences in study demographic variables between the intervention and control groups at baseline (). The number of patients decreased in weeks 5, 6 and 7 due to dose differences related to the treatment plan for each patient (). A total of six patients from both groups, (three in each group), were active smokers during the study.
Table II. Frequency of training from study start until 6 and12 months after completed radiotherapy (RT) and percent mean change in maximum interincisal opening (MIO) from baseline to 12 months after completed RT (n = 66).
There were no significant differences in MIO between the intervention and control groups at any of the measurement points (). During RT the intervention group had a mean decrease of 1.7% in MIO from baseline to week 4. By week 6 there was a 3.6% decrease in MIO from baseline. By three, six and 12 months after completed RT there was a 2.6%, 0.7%, and 0.3% decrease in MIO, respectively, compared to baseline. None of these differences within the group were statistically significant.
Figure 2. Mean change in MIO (%) from baseline, during RT and up to 12 months after completed RT. The lower number of patients weeks 6 and 7 is due to dose differences resulting in shorter time-period in external beam radiotherapy (see for details).
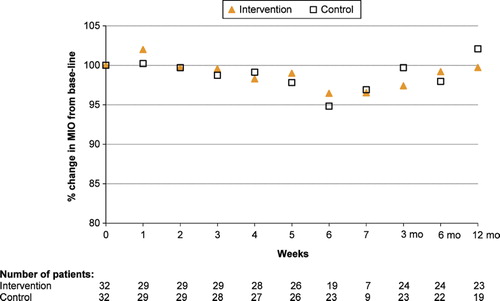
The control group had a 0.9% increase in MIO during RT at week 4 compared to baseline. Week 6 there was a 5.2% decrease in MIO. By three, six and 12 months after completed RT there was an increase of 2.8%, decrease of 1.7% and an increase of 4.1%, respectively, compared to the baseline levels. None of these differences within the group were statistically significant.
Four patients in the control group had at least a 15% decrease in MIO during week 2, 3, 5, and 6 of RT, respectively, and were therefore offered the same training programme as the intervention group (control/intervention group). These four patients had a 17% mean decrease in MIO by week 6 compared to baseline. By three months after completed RT there was a 12.6% increase in MIO compared to the six-week assessment. At month 6 and 12 there was a mean decrease in MIO compared to the three months measurement of 3.5% and 1.5%, respectively. Due to the small number of patients no formal statistical analyses were performed.
When investigating MIO in the different diagnosis groups, patients diagnosed with tonsil cancer had statistically larger mean MIO (48.6 mm, p = 0.012) at baseline compared to the rest of the HNC patients in the study (43.7 mm). This difference remained throughout the study.
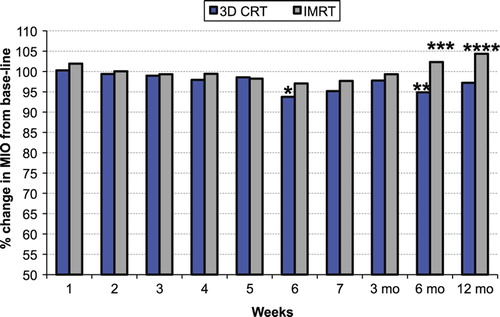
There was a statistically significant mean difference in MIO from baseline to week 6 (3 mm, p = 0.018) and month 6 (2.7 mm, p = 0.040) for patients receiving 3D-CRT (). Although the difference between patients treated with 3D-CRT and IMRT was 3.3 mm at month 6 (p = 0.065), and 3.4 mm (p = 0.084) at 12 months this was not statistically significant.
Figure 3. Mean change in MIO (%) from baseline, during RT and up to 12 months after completed RT. Patients with MIO decrease > 15%, who were offered training, is shown as a separate group. The lower number of patients weeks 6 and 7 is due to dose differences resulting in shorter time-period in external beam radiotherapy (see for details).
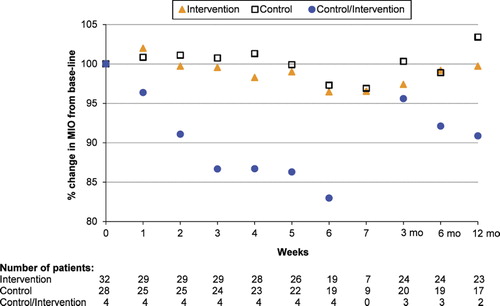
Figure 4. Mean change in MIO (%) from baseline, during RT and up to 12 months after completed RT in patients with 3D-CRT or IMRT. 3D conformal radiotherapy (3D-CRT) compared to baseline at 12 months:p = 0.22; *3D conformal radiotherapy (3D-CRT) compared to baseline at 6 weeks: p = 0.018; **3D conformal radiotherapy (3D-CRT) compared to baseline at 6 months: p = 0.040; ***3D conformal radiotherapy (3D-CRT) compared to IMRT at 6 months: p = 0.065; ****3D conformal radiotherapy (3D-CRT) compared to IMRT at 12 months: p = 0.084.
There were no significant differences in MIO between patients treated with RT and concurrent CT compared to RT only at baseline (p = 0.19), at three months (p = 0.14), at six months (p = 0.32). However there was a significant difference in patients MIO at 12 months (p = 0.033).
shows MIO in patients with different levels of training of the jaw muscles, both in the 42 patients who completed the entire study and in patients who dropped out (n = 24). Of the 24 patients that completed the study in the intervention group 18 patients trained 3–4 times daily up to the first six months. At 12 months, 12 patients from the same group decreased their training intensity. Although training was not part of the control group methodology, eight of 17 patients trained at different intensity up to 12 months after completed RT.
The group of four patients from the control who were offered the same training as the intervention group trained between three and five times a day. Of the six patients in the intervention group that trained 3–4 times a day there was a 4.3% decrease in MIO at 12 months. The nine patients from the control group that had no training had a mean increase of 1.7% in MIO at 12 months after completed RT. The eight patients from the control group that performed unsystematic stretching with different intensity had a greater increase in mean MIO at 12 months compared to the nine patients with no training.
Patients in the control/intervention group increased their mean MIO with 9.1% between week 6 and month 6, and increased their mean MIO with 7.9% between week 6 and month 12. However there was a mean decrease in their MIO with 1.2% between month 6 and month 12 ().
Discussion
This prospective randomised study shows that daily prophylactic training with TheraBite in HNC patients undergoing high dose RT does not affect mouth opening during RT or three, six and 12 months after completed RT. Also, this study shows a low incidence of RTIT in patients with HNC during RT or at three, six and 12 months after completed RT. However, the study indicates that a small group of patients are at risk of developing trismus early in the treatment phase and that they might benefit from training.
Patients in both the intervention group and the control group had a relatively small change in their MIO during RT and maintained their individual baseline MIO measurements at three, six and 12 months after completed RT. MIO changes were measured in percentual changes as this was clinically more relevant when taking the individuals normal variation in MIO into account. We expected to see a greater difference in MIO between the control group and the intervention group as all the known risk factors of developing RTIT were taken into consideration in the study design [Citation7,Citation8,Citation16–19].
Most of the patients in this study had high RT dosage (> 60 Gy) to the jaw muscles, which according to Goldstein [Citation7], is a major risk factor for a decrease in MIO. Our study found no significant change in MIO at three, six or 12 months in the control group compared with the intervention group related to dosage.
Tonsil [Citation19] and parotid gland [Citation17] cancer have been suggested as risk diagnosis groups for the development of RTIT, which are the main diagnosis groups in this study. One study reported a trismus incidence of 42% after completed oncological treatments over a 3–48-month time period. Our study reports on no significant difference in MIO between the main diagnosis groups.
According to the results of the present study, concurrent Cisplatin and RT does seem to have a diminishing effect on mouth opening in patients with HNC undergoing RT at 12 months after completed RT. These results are supported by another study [Citation8].
The low incidence of RTIT at three months (4%), at six months (4%) and at 12 months (4%) in our study was unexpected. According to Wang's [Citation20] retrospective findings, we expected to see at least a 7.2% decrease in MIO from baseline to three months in the control group, and at least a 14% decrease in MIO from baseline to six months. Wang's study also reports that trismus developed rapidly between one and nine months after RT, and then developed more slowly. Another prospective study showed RTIT incidence after completed RT to be 33% at three months, 38% at six months and 28% at 12 months [Citation19]. Some patients need to have reconstruction surgery to the mandible after RT or neck dissection and develop more severe trismus as a result of these procedures. However, these patients were not included in our study as they did not meet the inclusion criteria.
There was a small group of patients (n = 4) in the control group of this present study who performed no type of training and developed RTIT during RT (15% decrease in patients’ individual MIO). This subgroup of patients seems to be at greater risk of developing RTIT and may need to be identified early in the treatment phase so that they can be offered a stretching and training programme. By using TheraBite they increased their mouth opening by 12.6% at three months after completed RT, although there was a decrease of 3.5% in MIO noted from three to six months and a further decrease of 1.2% occurred between six and 12 months. These four patients had initially no type of training or stretching until they changed group, but there may be other quality of life factors affecting the development of RTIT. Based on the outcome of this study, these patients have a need for a training programme. However, the intensity and duration of the programme need to be further studied. TheraBite can be suggested as an exercise therapy to a smaller group of patients who are identified during RT with a 15% decrease in MIO. These patients were very motivated to train as there was an increase in MIO, resulting in compliance to the exercise programme. Future studies need to investigate if TheraBite is the best option or if there are other less costly training instruments/programmes.
Our study also reports on a normalisation of mouth opening in the control group at three, six and 12 months after completed RT, indicating that mouth opening exercise is not needed for the majority of patients with HNC undergoing RT. Kamstra et al. [Citation21] reported retrospectively on the effect of training with TheraBite when MIO was 35 mm or less and showed that patients who started training after three years from completed treatment had little effect of training.
The best results in mouth opening in our present prospective study were achieved by patients in the control group that stretched their jaw muscles randomly at least once daily compared to the intervention group, who trained mostly 1–4 times daily. These five patients in the control group, who did not adhere to control group methodology, acknowledged that the awareness of knowing that RT may result in a diminished MIO was enough to inspire a subconscious stretching of the jaw muscles. These patients stretched their jaw muscles at least once daily, without considering this to be a form of intervention. This may be a factor influencing the low incidence of RTIT in the control group, indicating a positive effect of daily stretching of the jaw muscles.
To our knowledge, no prospective well-designed studies have investigated different devices for training of mouth opening, but TheraBite [Citation22,Citation23] and Dynasplint Trismus System [Citation24], has been suggested in three small uncontrolled studies as exercise therapy for the increase of MIO.
The usage of IMRT treatment technique reduces normal tissue dosage and target coverage compared with 3D-CRT. Although our study shows only a few percent differences in MIO between the IMRT and 3D-CRT treatment groups, the results indicate that the IMRT technique gives a lower incidence of RTIT. It remains to be seen if these differences remain over time or if there is a cumulative effect in the incidence of RTIT. Our findings are consistent with the small retrospective study by Hsiung [Citation25].
Study strengths
This study is strengthened by design as a randomised study. Randomisation resulted in two homogeneous comparable groups (). A clear inclusion and exclusion criterion such as a MIO of over 35 mm from baseline ensures that this study only reports on RTIT and not on tumour-related trismus. No edentulous patients were included, which ensures a reliable method of MIO data collection. The individual's own normal variation in mouth opening was adhered to which gives a more accurate account of RTIT. The preceding pilot study strengthens the methodology and logistics of this study which led to a log book for the assessment of compliance. This study is further strengthened by its multicentre participation.
Study limitations
The rigid selection criteria can be argued as a limitation to the study as patients were excluded because of tumour-related trismus or omitting patients that already had trismus (< 35 mm) at the start of the study. However, these two factors can also be considered as strengthening the study. Poor physical function was assessed as an inability to comply with a training programme and follow-up which according to one study [Citation17] is a factor for the development of RTIT. Subsequently, this also highlights the challenge of investigating HNC patients receiving RT as does the high number of drop-outs (n = 24). Finally, different persons in the two measuring clinics performed the MIO measurements, although mouth opening can be measured reliably and should have no systematic effect on the outcome of this study [Citation26].
Conclusion
Patients with HNC undergoing high dose RT do not need to be burdened with an intense prophylactic training programme during RT and up to 12 months after completed RT. However, longitudinal MIO measurements during RT and up to12 months after completed RT are recommended to identify a small risk group who are an exception and may be in need of a training programme. Patients who have developed more severe RTIT should begin a more frequent training programme.
Future studies
Future prospective studies are needed to identify the smaller group of patients with HNC that are at risk of developing RTIT during RT and after completed RT. Quality of life factors need to be further investigated in the smaller risk group. Since the group of patients developing RTIT seems to be small, future studies need to have a multicentre cooperation. Also depending on the research question, less heterogenic groups need to be prospectively studied. The significant difference in MIO seen in this study between the IMRT group and the 3D-CRT cannot be explained in this study and needs further investigation.
Clinical implications/recommendations or guidelines
The results of this study indicate that all patients with HNC undergoing RT should be informed of possible changes in MIO during RT and up to one year after completed RT. Furthermore, there is a need of MIO baseline measurements and follow-up measurements in the management of patients with HNC undergoing high dose RT to identify patients at risk of developing more severe RTIT.
We suggest a long-term follow-up of MIO measurements up to 12 months after completed RT. This could be done by the hospital dentist during RT and follow-up, or by the patient themselves using a simple device measuring their mandibular range of motion. Patients who have developed more severe RTIT should begin a more frequent training programme, although the design and content of such a programme need to be further studied.
Acknowledgements
We thank the participating patients, the dentists and dental hygienists at the Department of Oral and Maxillo-facial Surgery in the south east region of Sweden for MIO measurements. All the professionals at the RT Departments in Linköping and Jönköping for their help in data collection. Lennart Andersson; dentist, Tomas Magnusson; Professor, Department of Oral Health Sciences, Jönköping University, and Håkan Rydberg for advice and support at the planning stages. Oncologists; Jan Rzepecki, Anna Koszewska-Flejmer, Måns Agrup, Christer Lindholm and Freddi Lewin, for help with identifying patients. Bengt Frost, for developing a data programme for data collection. This study has been funded by the Swedish Cancer Society, the Medical Research Council of Southeast Sweden, the Department of Radiation Oncology at the University Hospital in Linköping, the County Hospital in Jönköping, the Department of Medical and Health Sciences, division of Nursing Science, Linköping University, and the County Council of Östergötland. None of the authors have a financial relationship with the organisation that sponsored the research. All authors have full control of all primary data and agree to allow the journal to review data if requested.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Boyle P, Levin B, editors. World cancer report 2008. Lyon: ARC Press, International Agency for Research on Cancer; 2008.
- Socialstyrelsen. Cancer incidence in Sweden 2011. Cancerregistret. Stockholm 2012. [cited 2013 May 25]. Available from: http://www.socialstyrelsen.se/register/halsodataregister/cancerregistret
- . MeSH Browser [Internet]Holland JF, Bast RC, Morton DL, Frie III E, Kufe DW, Weichselbaum RR, editors. Cancer medicine. 4th ed. Baltimore: Williams & Wilkens; 1997. [cited 2013 May 25]; MeSH Unique ID: D006258. Available from: http://www.ncbi.nlm.nih.gov/mesh/?term = head+ and+ neck+ cancer
- Socialstyrelsen. Öppna jämförelser av cancersjukvårdens kvalitet och effektivitet. Socialstyrelsen. Stockholm 2011.
- Stenstrom Ling I, Larsson B. Individualized pharmacological treatment of oral mucositis pain in patients with head and neck cancer receiving radiotherapy. Support Care Cancer 2011;19:1343–50.
- Hancock PJ, Epstein JB, Sadler GR. Oral and dental management related to radiation therapy for head and neck cancer. J Can Dent Assoc 2003;69:585–90.
- Goldstein M, Maxymiw WG, Cummings BJ, Wood RE. The effects of antitumor irradiation on mandibular opening and mobility: A prospective study of 58 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:365–73.
- Jeremic G, Venkatesan V, Hallock A, Scott D, Hammond A, Read N, et al. Trismus following treatment of head and neck cancer. J Otolaryngol Head Neck Surg 2011;40:323–9.
- Velic E, Vreto A. Orala komplikationer i samband med radioterapi i huvud/hals regionen 2004. Report. Stockholm: Odontologiska institutionen, Karolinska institutet, Huddinge.
- Dijkstra PU, Kalk WW, Roodenburg JL. Trismus in head and neck oncology: A systematic review. Oral Oncol 2004; 40:879–89.
- Dijkstra PU, Huisman PM, Roodenburg JL. Critera for trismus in head and neck oncology. Int J Oral Maxillofac Surg 2006;35:337–42.
- Bensadoun RJ, Riesenbeck D, Lockhart PB, Elting LS, Spijkervet FK, Brennan MT. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer 2010;18:1033–8.
- Agerberg G. Maximal mandibular movements in young men and women. Swed Dent J 1974;67:81–100.
- Agerberg G, Österberg T. Maximal mandibular movements and symptoms of mandibular dysfunction in 70-year old men and women. Swed Dent J 1974;67:147–63.
- Vårdprogram. (2007). Munvård-medicinska aspekter, omvårdnad och rehabilitering. Stockholm-Gotlandregionen.
- Scott B, Butterworth C, Lowe D, Rogers SN. Factors associated with restricted mouth opening and its relationship to health-related quality of life in patients attending a Maxillofacial Oncology clinic. Oral Oncol 2008;44:430–8.
- Johnson J, Van As-Brooks CJ, Fagerberg-Mohlin B, Finizia C. Trismus in head and neck cancer patients in Sweden: Incidence and risk factors. Med Sci Monit 2012; 16CR278–82.
- Yeh SA, Tang Y, Lui CC, Huang YJ, Huang EY. Treatment outcomes and late complications of 849 patients with nasopharyngeal carcinoma treated with radiotherapy alone. Int J Radiat Oncol Biol Phys 2005;62:672–9.
- Pauli N, Johnson J, Finizia C, Andréll P. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol Epub 2012 Nov 29.
- Wang CJ, Huang EY, Hsu HC, Chen HC, Fang FM, Hsiung CY. The degree and time-course assessment of radiation-induced trismus occurring after radiotherapy for nasopharyngeal cancer. Laryngoscope 2005;115: 1458–60.
- Kamstra JI, Roodenburg JL, Beurskens CH, Reinstema H, Dijkstra PU. TheraBite exercises to treat trismus secondary to head and neck cancer. Support Care Cancer 2013; 21:951–7.
- Buchbinder D, Currivan RB, Kaplan AJ, Urken ML. Mobilization regimens for the prevention of jaw hypomobility in the radiated patient: A comparison of three techniques. J Oral Maxillofac Surg 1993;5:863–7.
- Cohen EG, Deschler DG, Walsh K, Hayden RE. Early use of mechanical stretching device to improve mandibular mobility after composite resection: A pilot study. Arch Phys Med Rehabil 2005;86:1416–9.
- Baranano CF, Rosenthal EL, Morgan BA, McColloch NL, Magnuson JS. Dynaslpint for the management of trismus after treatment of upper aerodigestive tract cancer: A retrospective study. Ear Nose Throat J 2011;90:584–90.
- Hsiung CY, Huang EY, Ting HM, Huang HY. Intensity-modulated radiotherapy for nasopharyngeal carcinoma: The reduction of radiation-induced trismus. Br J Radiol 2008;81:809–14.
- Jager-Wittenaar H, Dijkstra PU, Vissink A, Van Oort RP, Roodenburg JL. Variation in repeated mouth-opening measurements in head and neck cancer patients with and without trismus. Int J Oral Maxillofac Surg 2009;38:26–30.