To the Editor,
Hodgkin lymphoma (HL) may present with hepatic dysfunction, most often as a result of intra-hepatic HL involvement or bile duct obstruction caused by enlarged lymph nodes [Citation1]. In very rare cases, the disease is associated with paramalignant syndromes, such as idiopathic icterus or vanishing bile duct syndrome (VBDS).
VBDS is a severe condition that leads to the disappearance of small intrahepatic bile ducts. It has been associated with drugs, toxins and ischemia, and is a characteristic common feature of chronic rejection after liver transplantation. HL is a very rare cause of VBDS [Citation2]. Treatment depends on the cause and sometimes liver transplantation is necessary [Citation3].
We describe a case of HL-associated icterus considered secondary to VBDS, treated with BEACOPP- 14 and rituximab. The VBDS resolved eight months after the termination of the lymphoma treatment. The patient also underwent bilirubin apheresis and molecular adsorbing recirculating system (MARS) dialysis because of intractable pruritus.
Case report
In January 2008, the patient, suffering from abdominal pain and diarrhea, was diagnosed with suspected Crohn's disease. He had a previous hepatitis B infection and cholestatic liver values that continuously worsened. MRCP showed signs of possible sclerosing cholangitis. Liver biopsies demonstrated acute cholestasis and secondary toxic effects but no signs of primary sclerosing cholangitis (Supplementary Figure 1A, to be found online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2014.897001). In March 2008 the icterus worsened and intractable pruritus developed. Ultrasound and MRCP of the liver, bile ducts and pancreas was performed, as well as a second liver biopsy, gastroscopy and colonoscopy. The patient was positive for anti-HBc and anti-HBs but negative for HBs-antigen and HBV DNA. Due to positive CMV DNA with 1400–5700 copies/ml, he was treated with ganciclovir intravenously. A liver biopsy showed acute cholestasis, as well as centrilobular necrosis but no signs of CMV. The patient had enlarged lymph nodes on both sides of the neck and in the mediastinum.
A lymph node biopsy showed classical HL, of the nodular sclerosis subtype. No HL was seen in the liver, or in intestinal biopsies. VBDS secondary to the lymphoma was considered to be the underlying cause of the jaundice.
The lymphoma was staged with FDG-PET/CT, and found to be in stage IIB. He had four risk factors, according to the international prognostic score (male sex, anemia, low S-albumin and high leukocytes).
Treatment was initiated in April 2008 with bleomycin, etoposide, doxorubicine, cyclophosphamide, vincristine, procarbazine, prednisone and G-CSF (BEACOPP-14), with a 50% dose reduction of etoposide and cyclophosphamide, and a reduction to 60% of doxorubicine, due to the impaired hepatic function. He also received 375 mg/m2 of the anti-CD20 antibody, rituximab, four times weekly. The rationale behind the addition of rituximab was primarily to treat the jaundice of a paramalignant and possibly antibody-mediated origin. Furthermore, rituximab is known to have an anti-tumoral effect in HL even if the tumor cells are CD20-negative. Prior to the first chemotherapy course, the P-bilirubin level was 228 μmol/l. He received prophylactic treatment with lamivudine against hepatitis B reactivation, aciclovir against viral infections and trimetoprim-sulfa to prevent Pneumocystis jirovecii. Trimetoprim-sulfa was subsequently changed for inhalations with pentacarinat to avoid unnecessary liver toxic medication.
On admission for the second course of BEACOPP-14 the P-bilirubin was 407 μmol/l. His general condition was still poor. He had jaundice and severe itching. The doses of cyclophosphamide and doxorubicin were increased to approximately 70%. These doses were then maintained except a slight increase in the cyclophosphamide dose in courses 4–5.
After the second chemotherapy course, an FDG-PET/CT showed complete metabolic remission.
A third liver biopsy was performed after the second course of chemotherapy, with the specific question of VBDS development. This biopsy showed centrilobular damage and severe ductopenia, indicating VBDS. However, according to the definition of ductopenia, there should be a loss of interlobular bile ducts in at least 50% of the portal tracts, and more than 10 portal zones should be available for analysis [Citation4,Citation5]. In our patient, this liver biopsy contained four portal zones and bile ducts were found in only one (25%) of these. As the biopsy did not meet all the criteria for VBDS, one could challenge the diagnosis but the severe ductopenia and cholestasis in combination with the clinical features made the diagnosis of VBDS most likely.
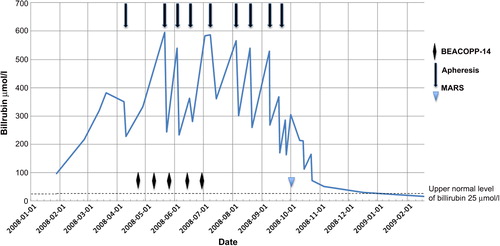
Due to intractable pruritus not responding to ordinary methods (antihistamines, ursodeoxycholic acids, rifampicin, venlafaxine and dermatology ointments and solarium treatments) we initiated bilirubin apheresis treatment with an anion-exchange adsorbent column (BR-350 Asahi Kasei Medical Corp.) for the reduction of bilirubin and bile acids. Plasma was separated by centrifugation using a COBE Spectra Apheresis System [Citation6]. Initially about 4000 ml of plasma was processed at each treatment. Later, the volume was increased to 6000 ml. The patient received 2–3 treatments on consecutive days every 2–3 weeks. The bilirubin reduction was between 30% and 40% after each set of treatment, and the corresponding figure for bile acids was similar (). Although the reduction in bilirubin was limited, the patient experienced a good symptomatic effect. At the end of the filtration sessions, we tried two MARS-dialysis sessions instead of using the bilirubin adsorbing column. This is a modified dialysis method using an albumin-containing dialysate that is recirculated and perfused online through charcoal and anion-exchanger columns. MARS enables the removal of bilirubin as well as albumin-bound substances [Citation7]. However, neither the reduction in bilirubin, or symptoms was considered better when compared with the previous treatments.
Figure 1. Bilirubin levels related to chemotherapy courses, bilirubin apheresis treatments and MARS dialysis.
Course 5 with chemotherapy was complicated with neutropenic sepsis. Blood cultures showed Klebsiella oxytoca. A short period of intensive care unit treatment was necessary. The chemotherapy was terminated after five of six planned courses.
After the termination of chemotherapy a new FDG-PET/CT showed complete metabolic remission. The P-bilirubin, however, was still high, thus apheresis was continued. Repeated liver biopsies after termination of treatment showed persistent acute cholestasis and ductopenia, with bile ducts in only 2/10 portal zones (Supplementary Figure 1B, to be found online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2014.897001). This indicated a persistent VBDS.
When admitted for an apheresis three months after the last course of chemotherapy and four months after the last course of rituximab, the patient was neutropenic (B-neutrophils < 0.1 × 109). This was interpreted as a late onset neutropenia due to the combination of chemotherapy and rituximab and the patient was treated with G-CSF successfully. A bone marrow biopsy did not show any HL engagement. As no improvement in liver function tests had been seen for eight months since the debut, a multidisciplinary team discussion about liver transplantation was held. The conclusion was to admit the patient for a pre-transplantation examination. No contraindications for transplantation were found but the decision was to await the development of the condition further.
Five months post-chemotherapy, a slow improvement in cholestasis was noticed. The patient experienced less pruritus, gained weight, and improved in general condition. The bilirubin level had decreased to 81 μmol/l, thus the apheresis was stopped.
Eight months after the lymphoma treatment was terminated, a normalized bilirubin was achieved. At this point, a fifth liver biopsy still showed the absence of bile ducts in some portal areas (Supplementary Figure 1C, to be found online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2014.897001) but the presence in other (Supplementary Figure 1D, to be found online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2014.897001) indicating reconstitution of regular bile ducts. In addition, mild acute cholestasis and secondary bile duct proliferation was observed. Sixty months after the termination of lymphoma treatment, the patient remains in complete remission and has completely normal liver function tests.
Conclusions
We describe a rare case of icterus considered to be caused by VBDS, associated with HL, treated with BEACOPP-14 and rituximab. The patient was also treated with apheresis and MARS dialysis. The VBDS spontaneously resolved eight months after termination of the lymphoma treatment.
In a review of idiopathic cholestasis and VBDS associated with HL, the number of described cases is stated to be 37, but only 19 had a liver biopsy that demonstrated VBDS [Citation8].
HL with associated VBDS and idiopathic icterus has a much poorer prognosis than HL in general, with a one-year overall survival and liver failure-free survival of 43% and 41%, respectively. Only 30% of patients have normal liver function at a median follow-up of 24 months. The only known possibility of recovering liver function is a complete remission of the lymphoma. Of patients achieving a complete remission, 60% ultimately achieve a normal liver function. On univariate analysis, early stage disease, complete remission of the HL, and radiotherapy are significantly associated with improved liver failure-free survival. The later may partly be explained by confounding factors such as higher staged HL in patients receiving chemotherapy alone. No patient recovered from impaired liver function without achieving a complete remission of HL [Citation8]. Thus, it seems to be crucial to give these patients the best lymphoma treatment possible. At least three previously described cases with HL and VBDS/IC were successfully treated with chemotherapy alone [Citation9–11].
Rituximab is an anti-CD20-antibody used to treat CD20-positive B-cell lymphomas. In HL, it is used to treat the rare nodular lymphocyte predominant subtype, in which the tumor cells are CD20-positive [Citation12]. Our patient, however, had the more common nodular sclerosis subtype, characterized by tumor cells that are CD20-negative. Some reports exist that rituximab also has a therapeutic role in these cases [Citation13] and this is explored in a German study (HD18). The rationale behind our decision to give this patient rituximab was instead to try to treat the presumed immunological reaction causing the VBDS.
The mechanism behind the association of HL and VBDS is not known, but different theories are proposed. A cell-mediated immunological reaction by cytotoxic T-lymphocytes, or toxic cytokines derived from the lymphoma cells may be responsible for the ductopenia [Citation8,Citation14,Citation15]. If it is antibody-mediated, rituximab could be a possible way of treating the mechanism directly, in addition to its possible anti-tumoral effect.
In our case, serial liver biopsies were analyzed. Initially the biopsies showed acute cholestasis and secondary toxic parenchymal changes, but no ductopenia, thus, not consistent with VBDS. In the biopsies taken three and five months after the index biopsy, ductopenia was obvious and VBDS was diagnosed. To our knowledge, this is the first case of HL-associated VBDS demonstrating the histopathological development and restitution of VBDS after successful Hodgkin treatment.
With apheresis treatment, our patient had a good symptomatic effect on the pruritus, even though the effect on the bilirubin levels was limited. The use of MARS dialysis in HL-related VBDS, has not previously been reported. Before receiving the MARS dialysis the patient´s P-bilirubin had decreased somewhat, but the improvement continued after the dialysis was terminated. Thus, the use of MARS dialysis in this setting has yet to be defined.
There are no publications concerning the time from successful lymphoma treatment to disappearance of the VBDS, i.e. how long you should wait until you start planning a liver transplant. In our case the patient remained icteric for eight months after termination of the treatment, and nine months after the first examination showing complete remission. This indicates that it is necessary to be patient and wait for spontaneous recovery after treating the lymphoma, since a liver transplant is a very expensive procedure carrying a large risk of serious complications. In some publications, the necessity of quick consideration of liver transplant is stressed [Citation3,Citation16,Citation17], but there are at least two previously reported patients with VBDS and HL, where post-therapy liver biopsies showed a regeneration of bile ducts after a complete remission of HL [Citation11,Citation18].
Supplementary Figure 1 (A–D)
Download PDF (2.8 MB)Acknowledgments
We wish to acknowledge all colleagues participating in the treatment of the patient, especially Anna Laurell and Magdalena Adde at the Department of Oncology and Karin Eklund, Eva Hillervik and Karin Mokvist at the Department of Medicine, Section of Nephrology, Uppsala University. Written informed consent was obtained from the patient for the publication of this Case Report and any accompanying images. A copy of the written consent is available for the review by the Editor-in-Chief of this journal.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bouroncle BA, Old JW, Jr., Vazques AG. Pathogenesis of jaundice in Hodgkin’s disease. Arch Intern Med 1962;110: 872–83.
- Reau NS, Jensen DM. Vanishing bile duct syndrome. Clin Liver Dis 2008;12:203–17, x.
- Hubscher SG, Buckels JA, Elias E, McMaster P, Neuberger JM. Reversible vanishing bile duct syndrome after liver transplantation: Report of 6 cases. Transplant Proc 1991;23: 1415–6.
- Ludwig J, Wiesner RH, LaRusso NF. Idiopathic adulthood ductopenia. A cause of chronic cholestatic liver disease and biliary cirrhosis. J Hepatol1988;7:193–9.
- Crawford AR, Lin XZ, Crawford JM. The normal adult human liver biopsy: A quantitative reference standard. Hepatology 1998;28:323–31.
- Geiger H, Klepper J, Lux P, Heidland A. Biochemical assessment and clinical evaluation of a bilirubin adsorbent column (BR-350) in critically ill patients with intractable jaundice. Int J Artif Organs 1992;15:35–9.
- Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, et al. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: Results of a prospective, randomized, controlled clinical trial. Liver Transpl 2000; 6:277–86.
- Ballonoff A, Kavanagh B, Nash R, Drabkin H, Trotter J, Costa L, et al. Hodgkin lymphoma-related vanishing bile duct syndrome and idiopathic cholestasis: Statistical analysis of all published cases and literature review. Acta Oncol 2008;47:962–70.
- Liangpunsakul S, Kwo P, Koukoulis GK. Hodgkin’s disease presenting as cholestatic hepatitis with prominent ductal injury. Eur J Gastroenterol Hepatol 2002;14:323–7.
- Leeuwenburgh I, Lugtenburg EP, van Buuren HR, Zondervan PE, de Man RA. Severe jaundice, due to vanishing bile duct syndrome, as presenting symptom of Hodgkin’s lymphoma, fully reversible after chemotherapy. Eur J Gastroenterol Hepatol 2008;20:145–7.
- Crosbie OM, Crown JP, Nolan NP, Murray R, Hegarty JE. Resolution of paraneoplastic bile duct paucity following successful treatment of Hodgkin’s disease. Hepatology 1997; 26:5–8.
- Maeda LS, Advani RH. The emerging role for rituximab in the treatment of nodular lymphocyte predominant Hodgkin lymphoma. Curr Opin Oncol 2009;21:397–400.
- Saini KS, Azim HA, Jr., Cocorocchio E, Vanazzi A, Saini ML, Raviele PR, et al. Rituximab in Hodgkin lymphoma: Is the target always a hit?Cancer Treat Rev 2011;37: 385–90.
- Hubscher SG, Lumley MA, Elias E. Vanishing bile duct syndrome: A possible mechanism for intrahepatic cholestasis in Hodgkin’s lymphoma. Hepatology 1993; 17:70–7.
- Strazzabosco M, Spirli C, Okolicsanyi L. Pathophysiology of the intrahepatic biliary epithelium. J Gastroenterol Hepatol 2000;15:244–53.
- Pass AK, McLin VA, Rushton JR, Kearney DL, Hastings CA, Margolin JF. Vanishing bile duct syndrome and Hodgkin disease: A case series and review of the literature. J Pediatr Hematol Oncol 2008;30:976–80.
- Rossini MS, Lorand-Metze I, Oliveira GB, Souza CA. Vanishing bile duct syndrome in Hodgkin’s disease: Case report. Sao Paulo Med J 2000;118:154–7.
- Cordoba Iturriagagoitia A, Inarrairaegui Bastarrica M, Perez de Equiza E, Zozaya Urmeneta JM, Martinez-Penuela JM, Beloqui Perez R. Ductal regeneration in vanishing bile duct syndrome in Hodgkin’s lymphoma. Gastroenterol Hepatol 2005;28:275–8.

