Abstract
Approximately 50% of patients with high-grade soft tissue sarcoma (STS) will develop pulmonary metastasis. This is the most frequent cause of death and improving treatment is warranted. Preoperative chemotherapy is used for selected patients, usually those with less favorable prognosis and mainly outside clinical trials. The predicted value of histological and radiological response to preoperative chemotherapy on outcome was the main focus for this investigation.
Patients and methods. This retrospective study comprises 93 patients with metachronous lung metastasis from STS who underwent complete metastasectomy alone (n = 41) or metastasectomy following preoperative chemotherapy (n = 52). Clinical data, histological and radiological responses to chemotherapy were recorded and survival analyses performed.
Results. The time from initial STS diagnosis to the appearance of metastasis was shorter in the preoperative chemotherapy group than in those treated with surgery alone (p = 0.02). However, no statistical differences in post-metastasis disease-specific survival (DSS) or progression-free survival (PFS) between the groups were demonstrated. Patients in the preoperative chemotherapy group with good (complete) histological response had improved PFS compared with poor responders (p = 0.04). Radiological partial response was an independent, favorable prognostic factor for improved PFS and DSS (p = 0.003).
Conclusion. Despite having unfavorable disease characteristics, some patients may benefit from preoperative chemotherapy. Both histological and radiological responses to preoperative chemotherapy seem to be prognostic in STS patients undergoing complete pulmonary metastasectomy.
Soft tissue sarcomas (STS) encompass a wide spectrum of rare tumors accounting for approximately 1% of all adult malignancies. The lungs are the most frequent metastatic site [Citation1]. With metastatic disease the prognosis is poor with a median survival of approximately 10 months [Citation2]. Overt metastasis at diagnosis (synchronous metastasis), metastatic relapse (metachronous metastasis) with a short interval from primary disease, more than three pulmonary nodules and bilateral involvement are related to inferior outcome [Citation3,Citation4]. Patients with long progression-free interval from diagnosis face a more favorable prognosis and selected patients can be cured if the metastases are completely resected [Citation2,Citation4]. Long-term survival may be feasible even with repeated metastasectomies [Citation4].
Nevertheless, more than 60% of patients undergoing pulmonary metastasectomy will experience subsequent recurrences due to co-existing micrometastatic disease [Citation4], and the strategy of eliminating such micrometastases by chemotherapy is tempting. Preoperative chemotherapy in patients with lung metastasis from osteosarcoma has resulted in approximately one third long-term survivors [Citation5]. This has led some to explore a similar approach also in STS [Citation2,Citation6]. Previous retrospective studies, however, have not shown improved outcome in patients with STS undergoing chemotherapy prior to resection of pulmonary metastases [Citation7,Citation8]. Furthermore, randomized trials in this setting have been difficult to conduct due to low enrollment [Citation9]. Importantly, two publications report long-term survivors among highly selected subsets of advanced STS patients with complete remission after surgery and/or chemotherapy (13 of 38 and 17 of 66 patients, respectively) [Citation10,Citation11].
In our institution preoperative chemotherapy has generally been reserved for patients < 70 years of age with good performance status and aggressive tumor biology; i.e. < 12 months metastasis-free interval (from diagnosis until first appearance of pulmonary metastasis), multiple pulmonary nodules, extrapulmonary- or bilateral pulmonary disease. Postoperative chemotherapy has been administered after radiological response evaluation according to WHO criteria [Citation12] and histological evaluation [Citation13]. Disease progression, poor histological response, or unacceptable toxicity often resulted in altered or no postoperative chemotherapy.
Radiological response evaluation in high grade STS has limitations due to stromal components that may keep the tumor volume unchanged post-treatment [Citation14]. This emphasizes the importance of histological evaluation of chemotherapy effectiveness. Well-defined histological criteria for assessing response have been established in osteosarcoma, and in this disease histological response has a clear relationship to outcome [Citation15]. To our knowledge, except one study [Citation10], this has only been examined in localized STS with disparate results [Citation10,Citation16–20].
This retrospective study includes all patients in our institution between 1980 and 2007 who underwent complete surgical removal of lung metastases occurring more than six months from first STS diagnosis. Attempts were made to address the impact of response to preoperative chemotherapy on long-term outcome.
Patients and methods
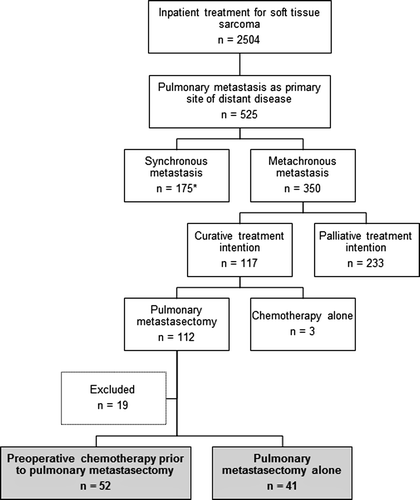
Searching the institution's Sarcoma Database identified 350 STS patients with metachronous pulmonary metastasis treated between 1980 and 2007 (). Among these, 112 were subjected to lung surgery following a metastatic relapse more than six months from their primary STS diagnosis. A retrospective review of the medical records was performed based on informed consent to sarcoma research in general obtained at time of primary diagnosis. Only patients with good performance status (ECOG 0-1), curative treatment intention and subsequent pulmonary metastasectomy were included. Reasons for exclusions were incomplete/palliative thoracoscopy/thoracotomy (n = 15), malignancy not conclusive (n = 2) and solely pleural metastasis (n = 2). This left a total of 93 patients for further analysis of survival, among which 52 had received preoperative chemotherapy and could be subjected to histological and radiological response evaluation (). The study was approved by the Institutional Review Board.
Response evaluation
Previously, histological response to preoperative chemotherapy in advanced STS has been divided into four grades based on the percentage of viable cells [Citation10]. The method was partly derived from the four-grade system defined by Huvos [Citation13] and was applied in the present study: Huvos grade I–II (I: no necrosis, II: < 90% necrosis) was considered poor, grade III (≥ 90% necrosis) intermediate and grade IV (100% necrosis) good histological response (i.e. complete). All specimens from patients treated with preoperative chemotherapy (except four cases with insufficient tissue for analysis) were histologically re-examined. The percentages of tumor necrosis were evaluated on routine slides stained with hematoxylin and eosin. All available slides were reviewed. If multiple metastases were resected and evaluated with variable histological responses (“mixed”), the definitive response was scored according to the poorest chemotherapy response.
Radiological response rates were classified according to standardized WHO criteria [Citation12]. Pre-chemotherapy measurements were done close to initiation of chemotherapy and pre-surgery measurements close to surgery. The number and dimension of metastases were determined based upon review of cross-sectional imaging studies (7.5–8mm CT slices) in lung window setting. When CT scans were not accessible (n = 18), plain x-rays were used. A maximum of five lesions were measured in each case. For single lesions multiplication of the longest diameter by the greatest perpendicular diameter was used. In multiple lesions the average of sum of products was applied. Relevant images were missing in seven cases, thus excluded from radiological analysis.
Principally, all preoperative evaluations were done by the institution's same multidisciplinary sarcoma team. Postoperatively, the patients were followed with radiological and clinical control every three months.
Statistical methods
The data were analyzed with respect to progression-free survival (PFS, i.e. next pulmonary metastasis, extrapulmonary metastasis or sarcoma related death) and disease-specific survival (DSS). Disease-free interval (DFI) was calculated from the date of primary diagnosis until first metastatic relapse, whereas PFS and DSS were defined from the date of first metastasis.
The statistical analysis was conducted using the software SPSS (version 16.0; SPSS, Inc., Chicago, IL, USA). Pearson's correlation test, Fisher exact test and independent two-tailed t-tests were employed to compare categorical and continuous variables, respectively. Factors previously reported to have prognostic effect on DSS and PFS were tested univariately with the Kaplan-Meier method, and the significance of detected differences estimated with log-rank test or Cox proportional hazard model. Changes were considered significant when p < 0.05.
Results
Patient and treatment characteristics
Among the 93 patients who underwent thoracotomy, 52 patients received preoperative chemotherapy (). When comparing these patients with the surgery alone group (41 patients), several differences were evident (): In the surgery alone group there were longer DFI, less bilateral disease and more females. There were no differences between the two groups as regards age, histological subtypes or additional presence of pleural- or extrapulmonary metastasis.
Table I. Comparison of clinicopathologic and treatment characteristics in study population.
Forty-two patients in the preoperative chemotherapy group were chemotherapy-naïve when lung metastasis occurred versus 30 in the surgery alone group. Several chemotherapy regimens were administered in the metastatic setting, but combinations of adriamycin and cyclo-/ifosfamide dominated (n = 32), then ifosfamide alone (n = 17) or other regimens (n = 3). In the latter groups, seven patients had received adriamycin adjuvantly. The median number of chemotherapy courses given was five (range 2–10). In the preoperative chemotherapy group 22 patients received postoperative chemotherapy. Five patients were allocated to a different chemotherapy regimen postoperatively due to either disease progression (n = 4) or unacceptable side effects (n = 1). Four patients in the no neoadjuvant group received postoperative chemotherapy.
Response to chemotherapy
Histology
Of 52 preoperatively treated patients, 48 cases were available for histological response assessments. The majority of these patients (n = 37) were poor responders to preoperative chemotherapy, but four were complete histological responders with no viable tumor tissue in the resected metastases.
Radiology
Forty-five cases were re-evaluated for radiological response to preoperative chemotherapy. One patient had no visible tumor [complete response (CR)], and this response was in accordance with the observation preoperatively and subsequent histological examination (fibrous scar with no evident tumor). All patients with radiological progressive disease (PD) were poor histological responders, while patients with partial response (PR) and stable disease (SD) displayed histological responses within all categories (). Consequently, there was no statistically significant correlation between radiological and histological chemotherapy responses.
Table II. Distribution of patients among histological and radiological response to preoperative chemotherapy.
Survival analysis
The median follow-up after first pulmonary metastasis was 39 months (range 4–241). Seventy-five patients died including 65 from sarcoma and three from postoperative complications related to thoracotomy (two lung embolisms and one postoperative arrhythmia). The other causes of death were one postoperative bleeding after brain metastasectomy, one from other cancers and five from unknown cause or other diseases. Of the remaining 18 patients in study population, two were alive with persistent disease while the 16 patients with no evidence of disease had a median follow-up of 117 (range 29–243) months from date of first metastasis. The median DSS for the entire study population was 41 months whereas the median PFS was 20 months.
Cumulative survival probability for study population, as illustrated in a, showed no statistical differences between the preoperative chemotherapy and surgery alone group (median DSS 38 months vs. 49 months and median PFS 17 vs. 25 months, respectively).
Figure 2. Kaplan-Meier curves depicting (a) DSS and PFS for the two cohorts in study population; preoperative chemotherapy and surgery alone. (b) PFS comparing various histopathologic responses to chemotherapy assessed by Huvos grading system and (c) DSS and PFS for various radiological responses; partial response (PR), stable disease (SD) and progressive disease (PD).
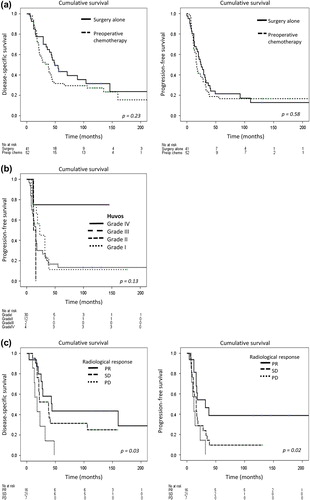
The histological response system showed that poorer response resulted in inferior survival with one important exception: Huvos grade III had inferior survival rate compared to Huvos grade I/II (b and see below). In contrast, patients with complete good histological response (Huvos grade IV, n = 4 of 48) were long-term survivors (three of four patients alive more than 10 years after the first metastasectomy) and with significantly longer PFS than the poor response group (median PFS for Huvos grade IV not reached vs. 16 months for Huvos grade I/II, p = 0.04). They also displayed longer PFS than the surgery alone group (p = 0.05). The impact of histological response on survival was significant in uni- and multivariate analyses where presence of Huvos grade IV improved progression-free survival together with unilateral pulmonary disease and absence of pleural metastasis. The histological subtype MPNST, however, was a significant adverse prognostic factor, as outlined in a. Albeit being a small group of tumors (n = 3), the group of long-term survivors included both presumed chemo-responsive (PNET, angiosarcoma) and less responsive (MFH) tumors with diverse disease aggressiveness (n = 1 unilateral pulmonary metastasis and DFI < 1 year) and extensiveness (n = 2 additional metastases).
Table III. A. Uni- and multivariate analysis in histological response groups. Progression-free survival.
A radiological PR or better also correlated significantly with survival (c), and patients with PR had median DSS of 44 months, whereas patients with SD or PD had median DSS of 38 and 18 months, respectively (p = 0.03). Median PFS was 32, 16 and 12 months, respectively (p = 0.02). In multivariate analysis PR improved both DSS and PFS significantly. In addition, PFS was favored by unilateral pulmonary disease whereas presence of MPNST, FS and local relapse were adverse prognostic factors (b).
Finally, patients who received additional postoperative chemotherapy (n = 22) had median DSS of 39 months and PFS of 18 months compared to 27 and 16 months with preoperative chemotherapy alone (n = 30). No statistically differences could be detected with the small sample sizes available. Similarly, no distinction could be observed between modified postoperative regimens and unchanged regimens (median DSS 21 vs. 45 months, median PFS 16 vs. 24 months, n = 5 vs. n = 17, respectively).
Discussion
STS in adult patients shows only moderate sensitivity to chemotherapy (e.g. 10% improvement in overall recurrence-free survival at 10 years following adjuvant doxorubicin-based chemotherapy [Citation21]), with lack of evidence for combination treatment of metastasis. In STS the strategy of eliminating micrometastases by chemotherapy is nevertheless tempting. This retrospective study investigates the impact of preoperative chemotherapy on pulmonary metastasis from STS in 52 patients compared with 41 patients treated with surgery alone. Since the patients were treated in a non-randomized manner, obviously there was a selection for more aggressive tumor biology [i.e. shorter time to first metastasis (DFI < 1 year) and bilateral disease] in the preoperative chemotherapy group. Interestingly, no differences in DSS or PFS could be demonstrated. This is in line with previous reports [Citation2,Citation7,Citation8], and may suggest an impact of chemotherapy in patients with presumed poor prognosis. There were no differences in age distribution or presence of pleural- or extrapulmonary metastasis between the two groups. This is in agreement with the report from Blackmon et al. [Citation22] which documented that long-term survival can be achieved if a complete resection is possible for both pulmonary and extrapulmonary metastasis.
Disparate responses are reported between primary tumor and metastasis in patients receiving preoperative chemotherapy [Citation23]. Thus radiological and histological chemotherapy response evaluation should be performed in the metastatic tissue. In the present study a significant concordance between radiological response in lung metastasis and outcome was found, and radiological partial response was one of the good prognostic factors for DSS and PFS. In contrast, radiological progressive disease was associated with dismal outcomes. Hence, additional chemotherapy and subsequent metastasectomies may be questioned in such patients.
A well-known histological response system (Huvos) was applied to evaluate preoperative chemotherapy response of pulmonary metastasis from STS. Poorer response resulted in poorer survival, with one important exception; Huvos grade III had inferior survival rate compared to Huvos grade I/II. Huvos grade III corresponds to more than 90% tumor necrosis, but still with foci of viable tumor present. To avoid interpreting any spontaneous necrosis as chemotherapy response, previous authors have argued that > 95% necrosis should be the cut-off for complete histological response [Citation19]. Thus, Huvos III may theoretically consist of more non-responding tumor than indicated by the response system. This might explain why there was no statistically significant correlation between radiological and histological chemotherapy responses.
In the present study only four patients were good histological responders according to the method applied. These patients turned out to be long-term survivors compared to the poor histological responders. Hence, good histological response to preoperative chemotherapy might be a surrogate marker of good clinical outcome. We recognize that the number of patients in this group is small, limiting the statistical power of the results. However, this group displayed diverse demography and disease extensiveness which emphasize the importance of not excluding patients with expected poor prognosis from chemotherapy, in line with a previous report [Citation11]. This observation is in contrast to some long-term survivors with indolent, lung only disease assumed to have fair prognosis only as a result of surgical complete metastasectomy [Citation4]. Hence, it might be presumed that complete surgical removal of all apparent disease partly explains the good prognosis observed in these patients, as does good histological response to preoperative chemotherapy.
One of the theoretical benefits of preoperative chemotherapy is the in vivo test of chemo-sensitivity. However, the gain in modifying postoperative chemotherapy regimen based on the histological response has been questioned for other tumor entities such as osteosarcoma [Citation15]. In the present study postoperative chemotherapy was administered after response evaluation in 22 preoperatively treated patients. This included all long-term survivors. Only five patients received new regimens postoperatively, four due to poor histological or radiological response evaluation. A trend towards increased disease- and progression-free survival by additional postoperative chemotherapy was found. However, no differences could be observed between modified postoperative regimens and unchanged regimens. Unfortunately, the sample sizes were too limited to conclude, but may raise the question of consolidation therapy, such as utilized in osteosarcoma, tentatively without changing regimens postoperatively. Larger studies are obviously needed to draw valid conclusions in this respect.
There are several limitations in the present dataset, primarily the selection bias inherent in the retrospective, non-randomized nature of this report with the selection for more aggressive disease biology in the preoperative chemotherapy group. However, patients with synchronous metastasis were excluded as these represent invariably poor prognosis (median DSS 22 months, PFS 12 months, data not shown). Hence, a cut-off of 6-months disease-free survival was selected. Furthermore, despite separate histological and radiological reviews, these were not blinded. Instead of using the prevailing RECIST criteria, less sophisticated imaging technology and WHO criteria were applied. This might lead to an overestimation of progressive disease [Citation24]. Finally, some patients were not chemotherapy-naïve at time of pulmonary metastasis. Although new drug regimens were administered, the effect might be outweighed by primary chemo-resistance.
In summary, although patients receiving preoperative chemotherapy had unfavorable disease characteristics, no difference in survival was observed compared to the surgery alone group. This might suggest an effect of chemotherapy. Furthermore, histological and radiological responses to such treatment seem predictive of survival. Based on this, selected patients may be offered pre- and postoperative chemotherapy with the aim to improve prognosis.
Acknowledgments
We thank Liv Aagedal and Trine Thoresen, The Norwegian Radium Hospital, for accurate updating the institution's Sarcoma Database, Dr. Wenche Reed and Unni Haakenaasen, The Norwegian Radium Hospital, for skillful pathological and radiological evaluation at the outset of this study and Dr. Are H. Pripp, Center for Biostatistics and Epidemiology, Oslo University Hospital, for statistical advice. The work was supported by the Norwegian Cancer Society and donations to the Norwegian Radium Hospital.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Brennan MF, Singer S, Maki RG, O’Sullivan B. Soft tissue sarcoma. In Cancer principles and practice of oncology. 7th ed. DeVita Jr VT, Hellman S, Rosenberg SA, editors. Philadelphia: Lippincott Williams and Wilkins; 2005. p 1581–637.
- Gadd MA, Casper ES, Woodruff JM, McCormack PM, Brennan MF. Development and treatment of pulmonary metastases in adult patients with extremity soft tissue sarcoma. Ann Surg 1993;218:705–12.
- Casson AG, Putnam JB, Natarajan G, Johnston DA, Mountain C, McMurtrey M, et al. Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 1992;69:662–8.
- Billingsley KG, Burt ME, Jara E, Ginsberg RJ, Woodruff JM, Leung DH, et al. Pulmonary metastases from soft tissue sarcoma: Analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999;229:602–10.
- Kager L, Zoubek A, Potschger U, Kastner U, Flege S, Kempf-Bielack B, et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol 2003;21:2011–8.
- Alvegard TA, Saeter G. The role of pulmonary metastasectomy for soft tissue sarcoma. Acta Orthop Scand Suppl 1997;273:145–7.
- Choong PF, Pritchard DJ, Rock MG, Sim FH, Frassica FJ. Survival after pulmonary metastasectomy in soft tissue sarcoma. Prognostic factors in 214 patients. Acta Orthop Scand 1995;66:561–8.
- Canter RJ, Qin LX, Downey RJ, Brennan MF, Singer S, Maki RG. Perioperative chemotherapy in patients undergoing pulmonary resection for metastatic soft-tissue sarcoma of the extremity: A retrospective analysis. Cancer 2007;110: 2050–60.
- van Geel AN, Blum RH, Alvegaard T, Baker LH. NCT00002764. EORTC-62933-SSG: Metastasectomy and chemotherapy for lung metastases from soft tissue sarcoma: A randomized phase III study (an intergroup study with the Scandinavian Sarcoma Group). 1997. Available from: http://www.clinicaltrial.gov
- Wiklund T, Saeter G, Strander H, Alvegard T, Blomqvist C. The outcome of advanced soft tissue sarcoma patients with complete tumour regression after either chemotherapy alone or chemotherapy plus surgery. The Scandinavian Sarcoma Group experience.Eur J Cancer 1997;33:357–61.
- Blay JY, van Glabbeke M, Verweij J, van Oosterom AT, Le CA, Oosterhuis JW, et al. Advanced soft-tissue sarcoma: A disease that is potentially curable for a subset of patients treated with chemotherapy. Eur J Cancer 2003; 39:64–9.
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207–14.
- Huvos A. Pathologic assessment of preoperative (neoadjuvant) chemotherapy. In: Bone tumors: Diagnosis, treatment and prognosis. 2nd ed. Philadelphia: W.B.Saunders Co.; 1991. p 122–8.
- Casper ES, Gaynor JJ, Harrison LB, Panicek DM, Hajdu SI, Brennan MF. Preoperative and postoperative adjuvant combination chemotherapy for adults with high grade soft tissue sarcoma. Cancer 1994;73:1644–51.
- Bacci G, Mercuri M, Longhi A, Ferrari S, Bertoni F, Versari M, et al. Grade of chemotherapy-induced necrosis as a predictor of local and systemic control in 881 patients with non-metastatic osteosarcoma of the extremities treated with neoadjuvant chemotherapy in a single institution. Eur J Cancer 2005;41:2079–85.
- Lucas DR, Kshirsagar MP, Biermann JS, Hamre MR, Thomas DG, Schuetze SM, et al. Histologic alterations from neoadjuvant chemotherapy in high-grade extremity soft tissue sarcoma: Clinicopathological correlation. Oncologist 2008;13:451–8.
- Schmidt RA, Conrad EU III, Collins C, Rabinovitch P, Finney A. Measurement and prediction of the short-term response of soft tissue sarcomas to chemotherapy. Cancer 1993;72:2593–601.
- Menendez LR, Ahlmann ER, Savage K, Cluck M, Fedenko AN. Tumor necrosis has no prognostic value in neoadjuvant chemotherapy for soft tissue sarcoma. Clin Orthop Relat Res 2007;455:219–24.
- Eilber FC, Rosen G, Eckardt J, Forscher C, Nelson SD, Selch M, et al. Treatment-induced pathologic necrosis: A predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol 2001;19:3203–9.
- Pezzi CM, Pollock RE, Evans HL, Lorigan JG, Pezzi TA, Benjamin RS, et al. Preoperative chemotherapy for soft-tissue sarcomas of the extremities. Ann Surg 1990; 211:476–81.
- Tierney JF, Stewart LA, Parmar MK. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: Meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 1997;350:1647–54.
- Blackmon SH, Shah N, Roth JA, Correa AM, Vaporciyan AA, Rice DC, et al. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long-term survival. Ann Thorac Surg 2009;88:877–84.
- Nachman J, Simon MA, Dean L, Shermeta D, Dawson P, Vogelzang NJ. Disparate histologic responses in simultaneously resected primary and metastatic osteosarcoma following intravenous neoadjuvant chemotherapy. J Clin Oncol 1987;5:1185–90.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada.J Natl Cancer Inst 2000;92: 205–16.