Abstract
Background. Giant cell tumor of bone (GCTB) is an aggressive primary osteolytic tumor. GCTB often involves the epiphysis, usually causing substantial pain and functional disability. Denosumab, a fully human monoclonal antibody against receptor activator of nuclear factor κΒ ligand (RANKL), is an effective treatment option for patients with advanced GCTB. This analysis of data from an ongoing, open-label study describes denosumab's effects on pain and analgesic use in patients with GCTB.
Material and methods. Patients with unresectable disease (e.g. sacral or spinal GCTB, or multiple lesions including pulmonary metastases) were enrolled into Cohort 1 (N = 170), and patients with resectable disease whose planned surgery was associated with severe morbidity (e.g. joint resection, limb amputation, or hemipelvectomy) were enrolled into Cohort 2 (N = 101). Patients received denosumab (120 mg) subcutaneously every four weeks, with additional doses on study days 8 and 15. Patients assessed worst pain severity with the Brief Pain Inventory – Short Form (BPI-SF) at baseline, at each visit for the first six months, and every three months thereafter.
Results. Clinically relevant pain improvement was reported by 29% of patients in Cohort 1 and 35% in Cohort 2 during week 1 and by ≥ 50% of patients in each cohort at each study visit from months 2–30. Median time to clinically relevant improvement was 30 (95% CI 16, 57) days in Cohort 1 and 15 (95% CI 15, 29) days in Cohort 2. Results in patients with moderate/severe pain at baseline were similar. Fewer than 30% of patients in Cohort 1 and 10% in Cohort 2 experienced clinically relevant pain worsening at any visit through 27 months. Most patients had no/low analgesic use during the study.
Conclusion. Most patients treated with denosumab experienced clinically relevant decreases in pain within two months.
Giant cell tumor of bone (GCTB) is an uncommon, primary osteolytic tumor, typically affecting the epiphyses of long bones of adults 20–40 years of age [Citation1–4]. Patients generally present with swelling, reduced joint movement, and increasing pain [Citation3,Citation5]. Pain may be activity related, caused by the loss of structurally significant bone and mechanical failure due to the tumor, or at rest, the result of tumor growth, tumor-associated expansion of the periosteum, and the response of the periosteum to the growing tumor [Citation3]. Although the tumor is classified as benign, it tends to be locally aggressive and can metastasize, most often to the lungs [Citation1–4].
Surgery is the treatment of choice for resectable tumors. En bloc excision is curative for primary lesions in 80% of cases [Citation4], but must be weighed against the need for reconstruction and associated complications, required revision surgeries, and possible recurrence [Citation1,Citation4]. When surgical resection is not feasible, radiotherapy is an option [Citation6,Citation7]. However, the potential for subsequent sarcomatous transformation, reported in about 2.5% of patients treated with megavoltage radiotherapy, is a concern [Citation3,Citation8,Citation9]. Given that the median time for malignant transformation after radiation treatment is thought to be 10 years, additional long-term follow-up is likely needed to evaluate the true frequency of this eventuality [Citation8,Citation9].
Discovery of receptor activator of nuclear factor κΒ ligand (RANKL) has contributed to the understanding of GCTB pathogenesis [Citation2,Citation4,Citation10]. RANK/RANKL signaling has been detected in three cell populations in GCTB tissue: mononuclear mesenchymal stromal cells, mononuclear osteoclast precursor cells, and multinucleated osteoclastlike giant cells [Citation2,Citation4,Citation10,Citation11]. Denosumab is a fully human monoclonal antibody against RANKL shown to inhibit osteoclast activity [Citation12] and significantly reduce or eliminate RANK-positive tumor giant cells [Citation11]. These effects inhibit the progression of GCTB, thereby improving clinical outcomes [Citation5,Citation13]. In an ongoing open-label, single-group, phase II study in 282 patients with GCTB, denosumab treatment was associated with objective tumor responses, prolonged disease stabilization and time to surgery, and reduced need for morbid surgery [Citation13]. On the basis of these findings, denosumab (Amgen Inc., Thousand Oaks, CA, USA) was recently approved in the US for the treatment of adults and skeletally mature adolescents with GCTB that is unresectable or where surgical resection is likely to result in severe morbidity. This study also assessed pain outcomes and analgesic use, which had not been systematically evaluated in GCTB patients previously. We now report the results of these endpoints that assessed pain and analgesic use among the patients enrolled in the ongoing phase II study (ClinicalTrials.gov Identifier: NCT00680992).
Material and methods
Patients
Interim safety and efficacy results of this study have been recently published [Citation13]. Briefly, adults or skeletally mature adolescents ≥ 12 years of age who had histologically confirmed GCTB with measurable evidence of active disease were enrolled in the study. Patients received denosumab (120 mg) subcutaneously at study visits every four weeks, with additional doses on days 8 and 15, for up to 30 months. Patients were advised to take daily calcium (≥ 500 mg) and vitamin D (≥ 400 IU) supplements. For the patient-reported outcome analyses, patients enrolled in two study cohorts were assessed. Cohort 1 comprised patients with unresectable disease (e.g. sacral or spinal GCTB, or multiple lesions including pulmonary metastases), and Cohort 2 comprised patients with resectable disease whose planned surgery was associated with severe morbidity (e.g. joint resection, limb amputation, or hemipelvectomy). Patients provided written informed consent before any screening procedures or investigational products were administered. The study was approved by the independent ethics committee or institutional review board at each center and was conducted according to applicable regulations and International Conference on Harmonisation Tripartite Guideline on Good Clinical Practice and applicable FDA regulations/guidelines.
Study procedures
Patients assessed their pain with the Brief Pain Inventory – Short Form (BPI-SF) at baseline and before treatment on days 8 and 15, every four weeks in months 1–6, and then every three months until the end of the study [Citation14]. Patients rated pain severity at its worst, at its least, on average, and now on an 11-point scale (0, no pain; 1–4, mild pain; 5–6, moderate pain; 7–10, severe pain) [Citation15]. The clinical relevance of changes in scores was determined on the basis of the minimally important difference (MID), the smallest difference that patients perceived as important and that would have led the clinician to consider a change in the patient's management [Citation16]. The MID for BPI-SF within this study was conservatively defined as 2 on the basis of anchor-based and distribution-based methods [Citation17,Citation18].
Analgesic use was determined at each visit from concomitant medication records and scored with the Analgesic Quantification Algorithm (AQA), an 8-point scale [Citation19]. AQA scores of ≤ 2 points were categorized as no/low analgesic use, and scores ≥ 3 points as strong opioid use.
Prespecified endpoints were the proportion of patients with a clinically relevant increase or decrease in worst pain (≥ 2-point increase or decrease from baseline, respectively) and the time to clinically relevant increase or decrease in worst pain (≥ 2 point increase or decrease from baseline, respectively), the proportions of patients with moderate or severe worst pain at baseline (worst pain score > 4 points) who shifted to no or mild pain (worst pain score ≤ 4 points) during the study, the proportions of patients with a shift from strong opioid use (score ≥ 3) to no/low analgesic use (score ≤ 2), and the proportion of patients with a shift from no/low analgesic use to strong opioid use.
Data analyses
Pain analyses included all patients who received at least one dose of denosumab and had at least one pain assessment. Statistical analyses were mainly descriptive, and no hypothesis testing was performed. Analyses were summarized for Cohort 1 and Cohort 2. The analyses were based on observed data. Kaplan-Meier methodology was used to estimate the time to pain improvement and worsening.
Results
Patients
A total of 170 patients in Cohort 1 and 101 in Cohort 2 were included in the analysis; over half the patients in each cohort were female. More patients in Cohort 1 than in Cohort 2 had recurrent disease (). The median age of the patients was 33 years in Cohort 1 and 34 years in Cohort 2. At baseline, moderate to severe pain was reported in 45% of patients in Cohort 1 and 49% in Cohort 2, and strong opioid use was reported in 33% of patients in Cohort 1 and 14% in Cohort 2.
Table I. Demographic and disease characteristics and pain and analgesic use scores at baseline.*
Pain improvement
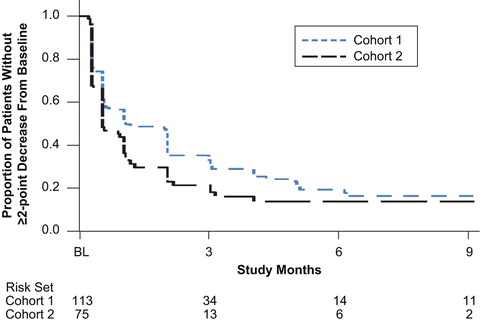
Pain reductions were similar in the two cohorts. Among the 113 patients in Cohort 1 and 75 patients in Cohort 2 with baseline BPI-SF pain scores ≥ 2, 29% in Cohort 1 and 35% in Cohort 2 had a clinically relevant decrease in pain during the first week, and 42% in Cohort 1 and 66% in Cohort 2 had a clinically relevant decrease in pain by month 1 (). At least half of the patients had a clinically relevant decrease in pain by month 2 in Cohort 1 and by month 1 in Cohort 2. The cumulative proportions of patients with a clinically relevant decrease in pain were similar in the two cohorts: 77% in Cohort 1 and 79% in Cohort 2. Kaplan-Meier estimates showed that the median time to a clinically relevant decrease in pain was 30 [95% confidence interval (CI) 16–57] days in Cohort 1 and 15 (95% CI 15–29) days in Cohort 2 ().
Figure 1. Proportion of patients who experienced a clinically relevant improvement in pain among patients with baseline pain scores ≥ 2. Time points with ≥ 10 patients are shown. n = number of patients with data at the visit.
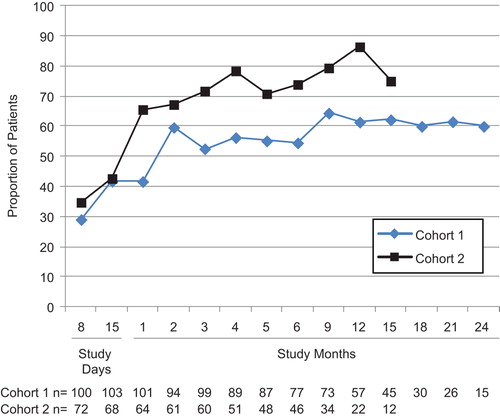
Figure 2. Time to clinically relevant improvement in baseline pain in patients with baseline BPI-SF pain score ≥ 2. Data shown through 9 months.
In the 75 patients in Cohort 1 and 47 patients in Cohort 2 with moderate or severe pain at baseline, 48% of patients in Cohort 1 and 80% in Cohort 2 had a shift from moderate or severe pain at baseline to no or mild pain by month 2.
Pain worsening
Of the 143 patients in Cohort 1 and 89 patients in Cohort 2 who had baseline worst pain scores ≤ 8, the proportions of patients who experienced a clinically relevant increase in pain score at any study visit ranged from 6.9% to 30% in Cohort 1 and from 0.0% to 8.2% in Cohort 2.
The estimated median time to clinically relevant pain worsening was 23.2 months for Cohort 1. The median time could not be estimated for Cohort 2 because few patients (16/89) experienced clinically relevant pain worsening during the study.
Changes in analgesic use
At any study visit, up to 39% of the 56 patients in Cohort 1 and up to 40% of the 14 patients in Cohort 2 who had strong opioid use at baseline reduced their analgesic use to no/low analgesic use. Of the 113 patients in Cohort 1 and 86 patients in Cohort 2 who had no/low analgesic use at baseline, ≤ 5.0% in Cohort 1 and ≤ 5.3% in Cohort 2 shifted to strong opioid use during the study.
Discussion
Denosumab treatment appears to be effective in decreasing pain in patients with both resectable and unresectable GCTB. Most patients in both cohorts experienced rapid and clinically relevant improvement in pain, and the proportion of evaluable patients experiencing improvement in pain was consistent across visits. A large majority of patients had no pain worsening during the study. Importantly, pain improvement did not appear to be associated with increased analgesic use, as fewer than 6% of patients in either cohort shifted to strong opioid use during the study. Our findings are consistent with those of an earlier phase II proof-of-concept study in which 26 of the 31 GCTB patients with baseline and post-treatment assessments had reduced pain or improved functional status with denosumab based on investigator report [Citation5].
The origin of pain in GCTB is multifactorial. In GCTB, nociceptors are activated by mechanical stress resulting from tumor-related pressure and tissue deformation, by the periosteal reaction to these stressors, and by production of prostaglandins, endothelins, and other noxious factors by the tumor cells [Citation20,Citation21]. GCTB-related pain may also be related to osteoclast-mediated acidification of the extracellular microenvironment resulting in depolarization of sensory neurons and transmission of pain signals to the spinal cord [Citation21]. Giant cells are responsible for the aggressive osteolytic activity observed in GCTB [Citation4,Citation5].
Denosumab-related osteoclast suppression reduces tumor-induced osteolytic activity, an effect which is manifested as substantial decrease in the metabolic activity of these tumors upon PET imaging [Citation13], as well as elimination of giant cells and replacement of neoplastic mesenchymal stromal cells with new woven bone upon histological analyses [Citation5]. On the basis of this mechanism of action, the drug would reasonably be expected to impede pain in patients with GCTB.
Several tools are available to measure patient-reported pain outcomes. In our study, the BPI-SF [Citation14], which has been shown to be a valid measure of pain in cancer and is one of the most widely used measurement tools for assessing pain, was used to assess pain severity. The BPI-SF is not specific to bone pain and therefore may also reflect non-GCTB-related pain. Thus, the BPI-SF may underestimate the effect of denosumab on bone pain. Further, the use of the single item “worst” is supported by the recommendations of the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) for assessing pain in clinical trials [Citation22]. We focused on individual responder analyses rather than changes in group means, consistent with the IMMPACT recommendations [Citation23], because they are easier for interpretation by clinicians.
To our knowledge, this is the largest prospective study of the effects of treatment on pain in patients with GCTB. While bisphosphonates have been used as treatment for GCTB, very little information, mostly from retrospective series and case reports, has been reported addressing the effect of these agents on pain in GCTB [Citation24–27]. In a retrospective case-control study, 24 patients with GCTB received perioperative treatment with intravenous and oral bisphosphonates and were followed for an average of 48 months. All patients reported a reduction in pain; the mean visual analog pain score improved from 7.7 to 3.3 [Citation25]. Similarly, little published information is available on pain response in patients with GCTB treated with radiotherapy. In a small case series, five patients with GCTB and pain and/or neurological deficits were followed for 30–107 months after intensity modulated radiotherapy (IMRT) [Citation28]. Of the four patients evaluable for clinical outcome, all had some level of symptom improvement [Citation28]. Additional study is needed to determine whether bisphosphonate and radiation treatment are effective in relieving pain associated with GCTB. With regard to radiotherapy, earlier reports indicated that most cases of secondary malignant GCTB appeared in irradiated patients [Citation29]. Although the use of newer megavoltage radiotherapy has reduced the incidence of this complication, it does still occur and therefore remains a concern [Citation9].
Conclusions based on the findings from this study are limited by its single-arm, open-label design. It is well established that patients may demonstrate a placebo response in randomized, placebo-controlled trials, although the reported size of this effect varies widely [Citation30–32]. We performed an uncontrolled study because there was no effective approved medical treatment available for GCTB, and evidence from the earlier phase II study suggested that denosumab may significantly benefit patients [Citation5]; use of a control group may have denied patients with unsalvageable GCTB access to effective treatment.
In our study, patients experienced rapid and clinically relevant pain relief. Reported adverse events were consistent with the known safety profile of denosumab [Citation13]. Together with the previously reported efficacy of denosumab in GCTB [Citation5,Citation13], these data suggest that denosumab represents an effective treatment option for patients with GCTB who have no or limited surgical options.
Declaration of interest: Medical writing support was provided by Amy Foreman-Wykert, PhD, an employee of Amgen Inc., and Mary Royer, a consultant to Amgen Inc. This work was supported by Amgen Inc. C. Cleeland is a consultant for Abbott, Bayer, Genentech, Amgen, and Exelixis. P. Glare has been a consultant for Amgen and Bayer and a speaker for Salix during the past 12 months. J. Engellau has participated in two expert panels on the use of denosumab and for these received an honorarium from Amgen. K. Skubitz has been a consultant for Amgen, Ariad/Merck, Novartis, Johnson & Johnson, Pfizer/Schering-Plough, Systems Medicine, and Seattle Genetics; owns publicly traded stock in Johnson & Johnson; has received research funding from Amgen, Novartis, GSK, Ariad/Merck, Celgene, Cell Therapeutics, Systems Medicine, Infinity, Schering-Plough, Bayer, Pfizer, and Daiichi; and provided expert testimony on the role of bisphosphonates in osteonecrosis of the jaw. A. Feng, Y. Qian, and C. Atchison are employees of Amgen and hold stock in the company. A. Braun, I. Jacobs, and K. Chung were previously employees of Amgen and held stock in the company. The remaining authors have no conflicts of interests to declare. As the sponsor, Amgen Inc. was involved in data collection, analysis of data, and assistance in the preparation of this manuscript. Publication decisions were made by the authors.
References
- Szendroi M. Giant-cell tumour of bone. J Bone Joint Surg Br 2004;86:5–12.
- Skubitz KM, Cheng EY, Clohisy DR, Thompson RC, Skubitz AP. Gene expression in giant-cell tumors. J Lab Clin Med 2004;144:193–200.
- Raskin KA, Schwab JH, Mankin HJ, Springfield DS, Hornicek FJ. Giant cell tumor of bone. J Am Acad Orthop Surg 2013;21:118–26.
- Thomas DM, Skubitz KM. Giant cell tumour of bone. Curr Opin Oncol 2009;21:338–44.
- Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, et al. Denosumab in patients with giant-cell tumour of bone: An open-label, phase 2 study. Lancet Oncol 2010; 11:275–80.
- Malone S, O’Sullivan B, Catton C, Bell R, Fornasier V, Davis A. Long-term follow-up of efficacy and safety of megavoltage radiotherapy in high-risk giant cell tumors of bone. Int J Radiat Oncol Biol Phys 1995;33:689–94.
- Shi W, Indelicato DJ, Reith J, Smith KB, Morris CG, Scarborough MT, et al. Radiotherapy in the management of giant cell tumor of bone. Am J Clin Oncol 2013;36:505–8.
- Chakravarti A, Spiro IJ, Hug EB, Mankin HJ, Efird JT, Suit HD. Megavoltage radiation therapy for axial and inoperable giant-cell tumor of bone. J Bone Joint Surg Am 1999;81:1566–73.
- Ruka W, Rutkowski P, Morysinski T, Nowecki Z, Zdzienicki M, Makula D, et al. The megavoltage radiation therapy in treatment of patients with advanced or difficult giant cell tumors of bone. Int J Radiat Oncol Biol Phys 2010;78:494–8.
- Atkins GJ, Kostakis P, Vincent C, Farrugia AN, Houchins JP, Findlay DM, et al. RANK Expression as a cell surface marker of human osteoclast precursors in peripheral blood, bone marrow, and giant cell tumors of bone. J Bone Miner Res 2006;21:1339–49.
- Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, Thomas DM, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res 2012;18:4415–24.
- Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 2004;19:1059–66.
- Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol 2013;14:901–8.
- Cleeland CS. Pain assessment in cancer. In: Osaba D, editor. Effect of cancer on quality of life. Boca Raton, Fl: CRC Press; 1991.
- Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 1995;61:277–84.
- Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR, Clinical Significance Consensus Meeting Group. Methods to explain the clinical significance of health status measures. Mayo Clin Proc 2002;77:371–83.
- Mathias SD, Crosby RD, Qian Y, Jiang Q, Dansey R, Chung K. Estimating minimally important differences for the worst pain rating of the Brief Pain Inventory-Short Form. J Support Oncol 2011;9:72–8.
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care 2003; 41:582–92.
- Chung KC, Barlev A, Braun AH, Qian Y, Zagari M. Assessing analgesic use in patients with advanced cancer: Development of a new Scale-The Analgesic Quantification Algorithm. Pain Med 2014;15:225–32.
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001;413:203–10.
- Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nat Rev Cancer 2002;2:201–9.
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19.
- Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146: 238–44.
- Balke M, Campanacci L, Gebert C, Picci P, Gibbons M, Taylor R, et al. Bisphosphonate treatment of aggressive primary, recurrent and metastatic Giant Cell Tumour of Bone. BMC Cancer 2010;10:462.
- Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: A case-control study. Bone 2008;42:68–73.
- Gille O, Oliveira Bde A, Guerin P, Lepreux S, Richez C, Vital JM. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine (Phila Pa 1976) 2012;37:E396–9.
- Chaudhary P, Khadim H, Gajra A, Damron T, Shah C. Bisphosphonate therapy is effective in the treatment of sacral giant cell tumor. Onkologie 2011;34:702–4.
- Roeder F, Timke C, Zwicker F, Thieke C, Bischof M, Debus J, et al. Intensity modulated radiotherapy (IMRT) in benign giant cell tumors – a single institution case series and a short review of the literature. Radiat Oncol 2010;5:18.
- Rock MG, Sim FH, Unni KK, Witrak GA, Frassica FJ, Schray MF, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am 1986;68:1073–9.
- Beecher HK. The powerful placebo. J Am Med Assoc 1955;159:1602–6.
- Turner JA, Deyo RA, Loeser JD, Von Korff M, Fordyce WE. The importance of placebo effects in pain treatment and research. JAMA 1994;271:1609–14.
- Linde K, Fassler M, Meissner K. Placebo interventions, placebo effects and clinical practice. Philos Trans R Soc Lond B Biol Sci 2011;366:1905–12.

