Abstract
It is debated whether cancer patients treated with chemotherapy can mount an adequate response to vaccination.
Material and methods. Ninety-six adult outpatients with cancer, who were undergoing chemotherapy and/or monoclonal antibody, tyrosine kinase inhibitor, irradiation or corticosteroid treatments, were studied. Two doses of the pandemic influenza A(H1N1)/09 AS03-adjuvanted split virion vaccine, one dose of the seasonal influenza vaccine and one dose of the 23-valent pneumococcal polysaccharide vaccine were given. Serum haemagglutination inhibition (HI) assays were used to determine antibody titres against the influenza strains. For the pneumococcal vaccine 14 different serotype-specific anti-capsular antibodies were measured by bead assay xMAP®.
Results. Patients treated with rituximab did not respond to vaccination. For patients without rituximab treatment 4% had putatively protective antibodies before vaccination (HI ≥ 40) to the pandemic-like strain A/California7/2009HINI. After the first and second dose of vaccine, seroprotection rates (SPR) were 62% and 87%, and seroconversion rates (SCR) 62% and 84%, respectively. Before seasonal flu vaccination SPR against influenza A/Brisbane/59/2007H1N1 and A/Uruguay/10/2007H3N2 were 19% and 17%, respectively. After vaccination, SPR were 70% and 59% and SCR 42% and 50%, respectively. For the pneumococcal vaccine protective antibodies were found to 40% of the 14 strains before and to 68% after vaccination. The mean response to pneumococcal vaccination was to 44% of the 14 serotypes. A response to at least 50% of the 14 serotypes was found in 49% of the patients. No serious adverse events were reported.
Conclusion. A substantial number of adult cancer patients with ongoing chemotherapy treatment could mount an adequate serological response to influenza and pneumococcal vaccination without severe adverse events. Thus, vaccination should be recommended. Adjuvanted vaccines may improve the vaccine response among this patient group. Patients recently treated with rituximab do not respond to vaccination.
Cancer patients are more susceptible to infections, partly due to the disease itself but also due to the treatments used [Citation1]. An infection can indirectly result in suboptimal cancer treatment by causing delays in treatment [Citation2].
Chemotherapy inhibits the immune system by suppressing bone marrow production of blood cells. As vaccines stimulate the very part of the immune system that chemotherapy suppresses, it is debated whether cancer patients treated with chemotherapy can mount an adequate response to vaccination [Citation3].
Influenza is a common, highly contagious, acute viral infection that causes annual epidemics across the world, typically occurring between December and April in Sweden. Immunocompromised cancer patients are reported to have an increased risk of contracting the influenza virus, with a higher risk of a more severe and prolonged disease [Citation2,Citation4–6]. In 2009 the novel influenza A(H1N1) was declared a full-scale pandemic by the World Health Organization (WHO). It contained a previously unknown, unique combination of gene segments from both North American and Eurasian swine lineages, partly derived from the virus that caused the worldwide 1918 pandemic flu with at least 50 million deaths (The Spanish flu) [Citation7].
Streptococcus pneumoniae is the leading cause of bacterial respiratory infection and is estimated to cause 1.6 million deaths annually worldwide, mostly among elderly individuals, children and immunocompromised individuals [Citation8].
The Swedish National Board of Health and Welfare (Socialstyrelsen) recommends vaccination against pneumococci and annual vaccination against seasonal influenza for high-risk groups including patients with malignancies receiving chemotherapy or other immune modulating therapies [Citation9]. In 2009 these recommendations also included vaccination against the pandemic influenza A(H1N1) 2009.
The aim of this prospective study was to determine the serological responses to these three recommended vaccines; the new AS03-adjuvanted influenza A(H1N1) 2009 vaccine, the non-adjuvanted seasonal trivalent influenza vaccine and the 23-valent pneumococcal polysaccharide vaccine in adult cancer patients. To the best of our knowledge this is the only study where the same individuals have been vaccinated with all three vaccines.
Material and methods
Patients and study design
In October and November 2009 cancer patients above 18 years of age treated at the Departments of Oncology at Uppsala University Hospital and Gävle County Hospital were asked to participate in the study. The study protocol was reviewed and approved by a regional ethics committee and each participant gave written informed consent according to institutional guidelines. The aim was to recruit as many patients as feasible, during the time period when mass vaccination against the 2009 pandemic influenza was ongoing. A relatively short inclusion period and limited supply of vaccine for some weeks reduced the number of participants enrolled in the study.
All outpatients with cancer, with ongoing treatments with chemotherapy, monoclonal antibodies, tyrosine kinase inhibitors or corticosteroids, were eligible for inclusion in the study. Exclusion criteria were known allergy against egg or other components in the vaccine, ongoing infection with fever, treatment with immunoglobulins for hypogammaglobulinaemia or a life expectancy of less than three months.
Medical records were reviewed and information regarding diagnoses, date of diagnoses, disease stage, previous cancer, ongoing oncological treatment at the time of each vaccination, ongoing steroid treatment, previous oncological treatment, intention of treatment and performance status (WHO score) was collected. Also noted was date of latest chemotherapy course preceding each vaccination.
Before vaccination commenced each participant completed a standardised questionnaire regarding occurrence of infections in the past year, previous severe infections, previous pneumococcal vaccination and current medication. Patients who had received pneumococcal vaccine within five years were not revaccinated. Baseline sera were obtained at day 0 before vaccination. No attempts were made to identify previous influenza vaccinations or infections.
Laboratory parameters [haemoglobin, white blood cells, neutrophils, platelets, and C-reactive protein (CRP)] and physiological parameters (pulse, blood pressure, and body temperature) were recorded prior to each vaccination.
Vaccination
Patients were given the influenza A(H1N1) 2009 AS03-adjuvanted split virion vaccine Pandemrix™ (GlaxoSmithKline) containing 3.75 μg of haemagglutinin antigen from the pandemic-like strain A/California/7/2009, the trivalent non-adjuvanted seasonal influenza vaccine Fluarix™ (GlaxoSmithKline) containing 15 μg haemagglutinin antigen from each of the strains A/Brisbane/59/2007, IVR-118 (H1N1), A/Uruguay/716/2007, NYMC X-175C (H3N2) (an A/Brisbane/10/2007-like virus) and B/Brisbane/60/2008 and the 23-valent pneumococcal polysaccharide vaccine Pneumovax™ (Sanofi Pasteur MSD).
At day 0 the first dose of the pandemic influenza A(H1N1) and the 23-valent pneumococcal polysaccharide vaccine was given. The second dose of the pandemic vaccine was given 3–4 weeks later [mean 25 ± 7 days (median 25, IQR 7)] and in most cases also the non-adjuvanted seasonal influenza vaccine. For some patients the seasonal influenza was given 3–4 weeks later when the sampling was done after the second dose of the pandemic influenza A(H1N1) vaccine. No patient received more than two vaccines simultaneously and the vaccines were always administered in different muscle groups to avoid an increase in local side effects.
All medical records were reviewed for one year from vaccination date to examine any post- vaccination occurrence of influenza or pneumococcal infection as well as late onset of adverse effects of the vaccines. For those participants who died during follow-up, the date and cause of death was recorded.
Serum collection
Serum was drawn prior to each vaccination and 4–6 weeks after each vaccination. The serum samples were stored at -70°C. All samples were coded and blinded and concomitantly sent to the laboratories.
Laboratory methods and measurement of immunogenicity
Influenza
The haemagglutinin inhibition (HI) test was performed, as described by the WHO criteria [Citation10], by GSK Biologicals on the influenza strain A(California/7/2009(H1N1) as antigen for the pandemic vaccine and A/Brisbane/59/2007(H1N1), A/Uruguay/716/2007(H3N2) and B/Brisbane/60/2008 as antigens for the seasonal influenza vaccine. The antigens were derived from egg-grown virus preparations and chicken erythrocytes were used. Non- specific inhibitors in the serum were removed via cholera filtrate. The patient sera were tested in duplicate and processed in two-fold serial dilution with a starting dilution of the treated serum of 1:10. The antibody titres are stated as the reciprocal value of the highest dilution that caused inhibition of the haemagglutinin reaction. Sera, whose titres were < 10, were assigned a titre of 5 for calculation purposes. Data are expressed as a geometric mean titre (GMT), seroprotection rate (SPR): the percentage of patients with a serum HI titre ≥ 40 and seroconversion rate (SCR): the percentage of patients developing at least a four-fold increase in post-vaccination titre or if the prevaccination titre was < 10 an increase ≥ 40. For B/Brisbane/60/2008 the B viruses were split with ether treatment while the A strains were not. Due to this treatment B titres are always higher than A titres and not comparable. Therefore only the SCR of the influenza B strain is shown.
Pneumococci
Anti-capsular polysaccharide IgG antibodies to serotypes 1, 3, 4, 6B, 7F, 8, 9N, 9V, 12F, 14, 18C, 19A, 19 F and 23F (Danish nomenclature) were measured by bead assay xMAP® Pneumococcal Immunity assay (Luminex®), according to the manufacturers’ instructions. WHO states > 0.35 μg/ml to be a threshold serum IgG antibody concentration for protection against invasive pneumococcal disease, at a population level, for all serotypes, measured by ELISA [Citation11]. The threshold concentration for protection against pneumonia is probably higher and likely varies between serotypes.
There are no defined protective IgG antibody concentrations for the assay used in this study, but antibody concentrations for serotypes 4, 9N, 18C and 19F measured with Luminex assay have shown correlation (correlation coefficient 0.61–0.76) with antibody concentrations obtained by conventional ELISA [Citation12]. Similar to other studies [Citation13,Citation14] we chose a two-fold or greater increase in antibody levels post-vaccination as an indicator of immune responsiveness together with IgG levels ≥ 1.0 μg/ml. This level was set putatively as a protective level in this study. A response to at least seven (50%) of the 14 pneumococcal serotypes was regarded as seroconversion response.
Statistical methods
The antibody results are shown as median and range or mean± standard deviation (SD) depending on what is most appropriate. Patients receiving chemotherapy, corticosteroids and/or rituximab were divided into subgroups for statistical analysis. Classification was based on the immunosuppressive properties of the cytotoxic drugs together with the amount of corticosteroids given. Chemotherapy regimens were scored 1–5 based on the probability of grade 3 or higher neutropenia or leukopenia, in accordance with the American National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events v3.0. Corticosteroid treatment was similarly scored 1–5 based on daily intake. Bolus doses of corticosteroids, given as anti-emetics during chemotherapy, were converted to the equivalent daily dose before scoring.
Patients were also divided into subgroups depending on the time interval between their latest chemotherapy course and vaccination. Patients vaccinated within seven days of chemotherapy treatment were compared with those vaccinated seven days before or after chemotherapy.
The responses to the vaccines were correlated with a number of clinical variables (current immunosuppressive treatment, age, WHO-score, neutrophil count, haemoglobin, CRP, cancer type, timing of vaccine in relation to chemotherapy, intent of treatment) using Spearman's rank order correlation test. Comparisons of proportions between groups were performed by χ2 analyses. Multivariate analysis was performed by logistic regression model, used to investigate the significant factors in the univariate analysis. Statistical tests were two-sided and p-value of < 0.05 were considered statistically significant. Analyses of vaccine response were performed using StatSoft® Statistica 10 software package. Demographic data were calculated using Windows® Excel program.
Results
Patients’ characteristics
In total 96 patients were recruited. Patient characteristics are listed in . The patients had in general a good performance status. The diagnosis of the cancer was breast 24, colorectal 22, prostate 13, gynaecologic 9, stomach 4, lung 3, brain 2, lymphoma 15 and one each of the following: cancer of unknown primary (CUP), oesophagus, head neck, kidney, malignant melanoma, pancreas, and sarcoma. Diagnoses and treatment regimens in detail are listed in Supplementary Table I available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.914243. Most of the patients (92%) had ongoing treatment with chemotherapy, monoclonal antibodies or tyrosine kinase inhibitors. The remaining patients had ongoing corticosteroid treatments, endocrine therapy or radiotherapy. Concomitant corticosteroid treatment with doses exceeding 10 mg prednisolone equivalent per day was administered to 16 patients (17%). The mean neutrophil count before vaccination of the first dose of the influenza A(H1N1) vaccine was 3.8 × 109/l (0.1–13.1), for the second dose influenza A(H1N1) 3.8 × 109/l (0.3–18.4), for the seasonal influenza vaccine 3.7 × 109/l (1.0–10.1) and for the pneumococcal vaccine 4.0 × 109/l (0.2–13.1). No patient had a history of splenectomy.
Table I. Baseline characteristics of the study population.
Due to deteriorating health or transfer to other hospitals or departments 10 patients discontinued the study prematurely. During the one-year follow-up period 32 patients died, 29 due to their cancer disease or causes not related to this study. For the remaining three patients, all in late stages of cancer, pneumonia was a contributing factor to death. One of these patients had an invasive pneumococcal pneumonia with positive blood cultures approximately one year after the pneumococcal vaccination. This patient had a minor response to the pneumococcal vaccine with a response rate of 36% to tested serotypes. For the other two patients no pathogen could be identified in cultures.
During the one-year follow-up, another patient had a probable pneumococcal pneumonia with a positive culture from a nasopharyngeal swab. The patient was treated with antibiotics and recovered from the infection. Unfortunately, no post-infection serum was available for this patient. Some other cases of less serious infections were detected but in none of these cases could infection with pandemic or seasonal influenza or S. pneumoniae be confirmed with laboratory tests. Since the one-year follow-up only included a review of medical records and no attempts were made to take serological tests, milder cases of infection could have occurred without our knowledge.
Overall, all vaccines were well tolerated and side effects were mild and transient, with the exception of a moderate allergic reaction to the influenza A(H1N1) vaccine. No case of narcolepsy was reported during the one-year follow-up.
Pandemic vaccine
Ninety-six patients were vaccinated with the influenza A(H1N1)/09 AS03-adjuvanted split virion vaccine. Immunogenicity results are shown in . Ninety-one (95%) of the patients were eligible for serological analyses after the first dose, and 88 (92%) of the patients after the second dose. Before vaccination, three patients (3%) had protective antibodies to A/California/7/2009(H1N1). After the first dose, 49 (54%) of the patients had HI-titres ≥ 40 and 48 (53%) had a seroconversion response (SCR) and after the second dose 66 (75%) of the patients reached seroprotection and 64 (72%) had a SCR.
Table II. Serological response to vaccination against Influenza A(H1N1) measured as HI-titres.
Seasonal influenza
Seventy-eight patients were eligible for seasonal influenza vaccination. shows the immunogenicity results for the influenza A strains A/Brisbane/ 59/2007(H1N1) (A/Bri) and A/Uruguay/10/2007 (H3N2) (A/Uru). Before vaccination 15 (19%) had HI-titres ≥ 40 to A/Bri and 13 (17%) to A/Uru. After vaccination 28 (37%) had a seroconversion response and 47 (60%) reached seroprotective levels for A/Bri and 33 (42%) and 42 (54%) for A/Uru, respectively. For the influenza B/Brisbane/60/2008 strain 34 (44%) had a seroconversion response.
Table III. Serological response to vaccination against seasonal influenza A/Brisbane/59/2007(H1N1) and A/Uruguay/10/2007(H3N2) as HI-titres.
Streptococcus pneumoniae
Fifteen of the 96 patients included in the study had a documented vaccination against S. pneumoniae recently (less than five years ago) and were not given the pneumococcal vaccine. The remaining 81 patients were vaccinated with the 23-valent pneumococcal polysaccharide vaccine. Because of logistic reasons, serum analysis from 14 patients could not be performed. Serological analyses could thus be performed in 67 patients before vaccination and 63 patients after vaccination.
The responses to vaccination and SPRs to the 14 different pneumococcal serotypes before and after vaccination are shown in the and and in Supplementary Table II, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.914243.
Figure 1. Number of pneumococcal serotypes to which patients showed serotype-specific protective antibodies before and after vaccination, respectively. Horizontal bars represent the mean number of serotypes, symbols represent individual patients (n = 63). The total number of serotype specificities analysed was 14.
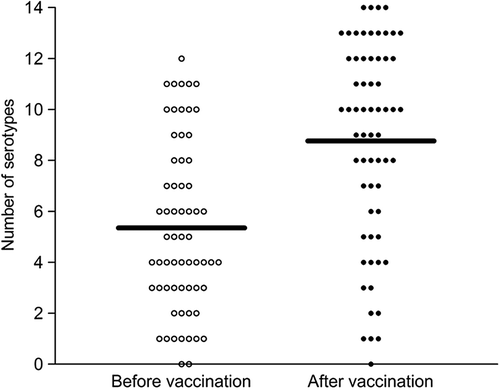
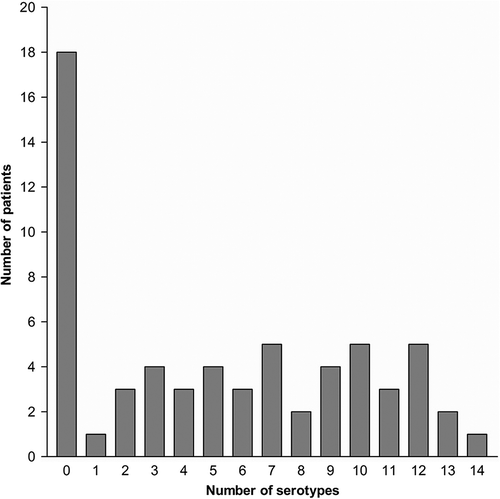
Figure 2. Number of patients (total n = 63) developing seroconversion to different numbers of pneumococcal serotypes. Among the 18 non-responder, 8 had received rituximab.
Before vaccination 67 patients had serotype- specific protective pneumococcal antibodies (SPP) to 5.4 (39%)± 3.3 (SD) of the 14 pneumococcal serotypes and after vaccination the 63 patients with available sera had SPP to 8.8 (63%)± 3.9 (p < 0.0001) serotypes (). Twenty-one (31%) of 67 patients had protection to at least seven (50%) of the 14 pneumococcal serotypes before vaccination and after vaccination this level of protection was reached by 46 of 63 patients (73%, p < 0.001, χ2-test).
The mean serotype response to pneumococcal vaccination (RPV) for all 63 patients was to 5.4 (39%)± 4.6 of the 14 different pneumococcal serotypes.
Twenty-seven (43%) of the 63 patients responded to at least seven (50%) of the 14 pneumococcal serotypes and this was regarded as seroconversion response to pneumococcal vaccine (). Some patients had antibody levels below or above the standard curve of the assay. Thus, the true number of patients with response to pneumococcal vaccination may have been underestimated.
Subgroup analyses
To further analyse the responses to vaccination the patients were subgrouped into patients with lymphoproliferative malignancies who were treated with the monoclonal antibody rituximab within six months (n = 13) and remaining patients not treated with rituximab (n = 83). Subgroup analysis showed that of the 13 patients treated with the monoclonal antibody rituximab only one responded to vaccination against influenza A(H1N1) and none responded to vaccination against seasonal influenza. For the patients not treated with rituximab 63 (84%) had a seroconversion response and 65 (87%) reached seroprotection level of antibodies after the second dose of the influenza A(H1N1) vaccine. Regarding the seasonal influenza vaccine 28 (42%) of the patients not treated with rituximab had a seroconversion response and 46 (70%) reached seroprotective levels for A/Bri and 33 (50%) and 39 (59%) for A/Uru, respectively.
Eight patients were treated with rituximab within six months of vaccination with the pneumococcal vaccine. Prior to vaccination these patients had SPP to 3.8 (27%)± 3.8 of 14 pneumococcal serotypes and after vaccination they showed SPP to 3.6 (26%)+ 4.0 (NS). None had a RPV to any of the 14 different pneumococcal serotypes. The 55 patients who were not treated with rituximab had SPP to 5.6 (40%)± 3.2 serotypes before and to 9.5 (68%)± 3.3 serotypes after vaccination, the mean RPV was to 6.2 (44%)± 4.4 of the 14 pneumococcal serotypes and 27 (49%) had a SCR to pneumococcal vaccine. In comparison between the eight rituximab-treated patients and 55 non-treated, the mean SPP was not significantly different before vaccination whereas it was significantly increased after vaccination for those who did not receive rituximab (p < 0.001) and also the RPV differed significantly (p < 0.001).
Approximately 50% of the patients were vaccinated within seven days. Analysis of timing of vaccination in comparison to chemotherapy showed no immediate difference between patients receiving vaccination within seven days of chemotherapy treatment compared to patients receiving vaccination > 7 days separate of chemotherapy.
The patients’ corticosteroid consumption were recorded and categorised over time. The median daily consumption was 15 mg prednisone (range 0–70 mg). The patients were also categorised based on the risk of their chemotherapy treatment causing grade III–IV neutropenia and leukopenia. Univariate and multivariate analyses showed only a slight trend to lower response to vaccination with higher doses of steroids and more intensive chemotherapy treatment (data not shown). However, some patients received adequate immunological response to the vaccination despite of high doses of steroids and intensive chemotherapy treatment.
Further subgroup analyses concerning cancer diagnosis or treatments were inconclusive due to lack of statistical power (data not shown).
There were no significant differences in the SCRs between the age groups < 60 years of age, 60–69 years of age or ≥ 70 years of age for any of the vaccines. However, in patients not treated with rituximab, the antibody titres (GMT) were significantly higher in patients younger than 60 years after two doses of the pandemic vaccine (p < 0.04).
Discussion
This prospective study evaluated the immunogenicity of vaccination against influenza A(H1N1) 2009, seasonal flu and S. pneumoniae in adult cancer patients. Previous studies for patients with solid tumours did not include all three vaccines. Many of them included fewer patients (n = 25–67) [Citation15–21]. Two studies did include more patients (n = 146 and 197) but in those studies only a fraction [n = 38 (26%) and 68 (35%)] of the individuals were undergoing chemotherapy treatment during the course of vaccinations [Citation22,Citation23]. Many of the studies were limited to fewer cancer diagnoses or cancer treatments [Citation15,Citation17–19,Citation21]. Our study consisted of a heterogeneous population representing a typical outpatient constellation in a department of oncology.
In otherwise healthy adults vaccination with the pandemic vaccine used in this study has shown a 98% seroprotection and a 98% SCR after one dose of vaccine [Citation24]. Data from a meta-analysis concluded that adequate seroprotection against pandemic influenza A(H1N1) was achieved in all age groups, except children younger than three years of age, after one dose [Citation25]. Our results indicate that cancer patients with ongoing treatment respond to vaccination but to a lesser degree. The improved response after the second dose indicates that at least two doses of the adjuvanted vaccine seem to be preferable in immunocompromised patients.
The vaccine against seasonal flu is reported to give a 70–90% protection against clinical disease in healthy adults [Citation26]. For cancer patients included in this study the antibody response was lower than what would be expected in healthy individuals. This is consistent with other studies investigating the effect of vaccination in cancer patients [Citation16–19,Citation21]. The general antibody responses against the different influenza strains in the seasonal flu vaccine were lower compared to the responses to the pandemic vaccine which might be due to the squalene-based adjuvant used in Pandemrix™, triggering the immunological response to a higher degree. Although only one dose of the non-adjuvanted seasonal influenza vaccine was given, previous studies have not shown any booster effect with repeated doses with this vaccine in immunocompromised patients [Citation27].
In this study we used the commercial bead assay xMAP® Pneumococcal Immunity assay (Luminex®) to measure the serotype-specific pneumococcal vaccine response. The xMAP® is less time consuming to perform and requires smaller sample volumes compared with the WHO-standardised ELISA. The disadvantage of the bead assay is that information regarding the serotype-specific antibody concentrations correlated with protection against pneumococcal infection has been produced with the WHO-standardised ELISA. However, the two methods have shown acceptable correlation according to some studies [Citation12], albeit with slightly higher values obtained with the bead assay. Also, in recent years the bead assay has been employed in several studies. This has provided information on antibody concentrations in different control and patient groups for reference. The vaccine against S. pneumoniae has been reported to provide 60–70% protection against invasive pneumococcal disease [Citation28]. In our study vaccination against pneumococci in adult cancer patients generated a lower serological response compared to a normal population, however, several patients did mount an adequate response.
The response rate among the study population was negatively influenced by the lack of response among rituximab-treated patients. The antibody rituximab is a chimeric monoclonal antibody directed against CD20 + surface antigens on B-lymphocytes. It is an effective treatment for CD20-positive B-cell lymphoma, alone or in combination with chemotherapy. Rituximab induces a rapid and prolonged depletion of CD 20 + B-lymphocytes and thus targets the key cells to successful immunisation [Citation29,Citation30]. In this study the response to vaccination in patients treated with rituximab was extremely poor indicating that vaccination during ongoing treatment is futile. Vaccination should take place prior to the start of rituximab treatment or 6–8 months after the last administered dose. Similar results are confirmed in other studies [Citation15,Citation19].
Systemic corticosteroid therapy is commonly used in the treatment for cancer either in a transient period during chemotherapy and/or as palliative maintenance treatment. Corticosteroids have a broad effect on immune function. It may increase the risk of infection, especially in doses exceeding 10 mg prednisolone per day [Citation31,Citation32]. This study showed a tendency to lower response rate, even though this could not be statistically verified, and there were also some patients treated with doses of 40 mg prednisolone who mounted an adequate antibody response.
The timing of vaccination in relation to chemotherapy may also have an impact on response to vaccination. It has been proposed that vaccination in mid-cycle is preferable instead of vaccination concomitantly with administration of chemotherapy [Citation6]. In a study of patients with breast cancer treated with FEC there was a tendency to better responses when vaccination was performed early during the chemotherapy cycle [Citation18]. In our study some patients received the vaccine on the same day as chemotherapy and some between courses. No differences in immunological responses were seen. However, more studies need to be conducted to further investigate this matter.
As the pandemic influenza period came to an abrupt end in Sweden at the end of 2009 and beginning of 2010 and epidemiological surveillance systems showed no other seasonal influenza epidemics during that season [Citation33], we find it likely that the antibody responses obtained truly represent a response to vaccination and not a result of an infection. In this study the vaccines were well tolerated with few side effects and there were very few infections during the follow-up period of one year, indicating that vaccination seems to be safe for this group of immunocompromised patients. Further on, in spite of the lower immunological responses and in spite of the fact that many of the patients had been treated with several previous lines of chemotherapy the response rates were not less than approximately 40% for any of the used vaccine. Although the measurement of serological response to vaccination is a surrogate marker for protection against infection our results support that these vaccines should be used in this patient group and that the used vaccines seems to be safe.
A French study from 2008 [Citation34] showed a vaccine coverage of 30% in adult cancer patients. Our patients had even lower vaccination coverage of 16% prior to inclusion in the study. Given the results of this study, information on the benefits of vaccination to chemotherapy-treated cancer patients should be spread in order to reduce morbidity and mortality in influenza and pneumococcal infections.
In conclusion, vaccination against influenza A(H1N1) 2009, seasonal flu and S. pneumoniae induces lower antibody response in adult cancer patients compared to healthy adults. Even though the serological response rates were lower, a substantial proportion of these patients developed protective antibody responses indicating that routine vaccinations should be recommended in adult cancer patients undergoing immunosuppressive chemotherapy regimes. The vaccinations were safe. When vaccinating against the pandemic influenza A(H1N1) 2009 two doses of the adjuvanted vaccine should be administered. An adjuvanted vaccine seems to improve the response to vaccination in cancer patients and if available such a vaccine should be preferred in these patients, but with regard to adverse effects. Patients with ongoing treatment with rituximab do not mount a serological response to vaccination; they should therefore be vaccinated before rituximab treatment starts or 6–8 months after the last administered dose. There were trends towards lower responses in patients treated with high-dose corticosteroid treatment. The timing of vaccination in relation to chemotherapy did not seem to influence the serological response rate.
http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.914243
Download PDF (34.3 KB)Declaration of interest: K. Pauksens has performed clinical trials for GlaxoSmithKline, Pfizer, Eurocine Vaccines, Sanofi Pasteur. GlaxoSmithKline Biologicals performed the assays for measurement of antibody response to influenza vaccination (all sera were blinded).
References
- Brydak LB, Marchala M. Humoral immune response to influenza vaccination in patients from high risk groups. Drugs 2000;60:35–53.
- Feldman S, Webster RG, Sugg M. Influenza in children and young adults with cancer: 20 cases. Cancer 1977;39:350–3.
- Sommer AL, Wachel BK, Smith JA. Evaluation of vaccine dosing in patients with solid tumors receiving myelosuppressive chemotherapy. J Oncol Pharm Pract 2006;12:143–54.
- Kempe A, Hall CB, MacDonald NE, Foye HR, Woodin KA, Cohen HJ, et al. Influenza in children with cancer. J Pediatr 1989;115:33–9.
- Yousuf HM, Englund J, Couch R, Rolston K, Luna M, Goodrich J, et al. Influenza among hospitalized adults with leukemia. Clin Infect Dis 1997;24:1095–9.
- Ortbals DW, Liebhaber H, Presant CA, Van Amburg AL 3rd, Lee JY. Influenza immunization of adult patients with malignant diseases. Ann Intern Med 1977;87:552–7.
- Morens DM, Taubenberger JK, Fauci AS. The persistent legacy of the 1918 influenza virus. N Engl J Med 2009; 361:225–9.
- Prato R, Tafuri S, Fortunato F, Martinelli D. Why is it still important that countries know the burden of pneumococcal disease. Hum Vaccin 2010;6:918–21.
- Recommendations of the Swedish National Board of Health and Welfare:Influenza vaccination. (Socialstyrelsens allmänna råd. Vaccination mot influenza. SOFS1997:21. [cited 2012 Oct 25]. Available from: http://www.socialstyrelsen.se/sosfs/1997-21.
- Hehme NW, Künzel W, Petschke F, Türk G, Raderecht C, van Hoecke C, et al. Ten years of experience with the trivalent split-influenza vaccine, Fluarix. Clin Drug Invest 2002;22: 751–69.
- World Health Organization: The immunological basis for immunization series.Immunological basis for immunization: Pneumococcal vaccine.2009. WHO/IVB/ISBN978924 1598217.
- Borgers H, Moens L, Picard C, Jeurissen A, Raes M, Sauer K, et al. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin Immunol 2010;134:198–205.
- Hartkamp A, Mulder AH, Rijkers GT, van Velzen-Blad H, Biesma DH. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine 2001;19:1671–7.
- Shildt RA, Rubin RR, Schiffman G, Giolma P. Polyvalent pneumococcal immunization of patients with plasma cell dyscrasias. Cancer 1981;48:1377–80.
- Yri OE, Torfoss D, Hungnes O, Tierens A, Waalen K, Nordøy T, et al. Rituximab blocks protective serologic response to influenza A(H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood 2011;118:6769–71.
- Mackay HJ, McGee J, Villa D, Gubbay JB, Tinker LM, Shi L. Evaluation of pandemic H1N1 (2009) influenza vaccine in adults with solid tumor and haematological malignancies on active systemic treatment. J Clin Virol 2011;50:212–6.
- Nordøy T, Aaberge IS, Husebekk A Samdal HH, Steinert S, Melby H, et al. Cancer patients undergoing chemotherapy show adequate serological response to vaccinations against influenza virus and Streptococcus pneumoniae. Med Oncol 2002;19:71–8.
- Meerveld-Eggink A, de Weerdt O, van der Velden AM, Los M, van der Velden AW, Stouthard JM. Response to influenza virus vaccination during chemotherapy in patients with breast cancer. Ann Oncol 2011;22:2031–5.
- Bedognetti D, Zoppoli G, Massucco C, Zanardi E, Zupo S, Bruzzone A, et al. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non-Hodgkin's lymphoma patients treated with rituximab- containing regimens. J Immunol 2011;186:644–55.
- Rousseau B, Loulergue P, Mir O, Krivine A, Kotti S, Viel E. Immunogenicity and safety of the influenza A H1N1v 2009 vaccine in cancer patients treated with cytotoxic chemotherapy and/or targeted therapy: The VACANCE study. Ann Oncol 2012;23:450–7.
- Loulergue P, Alexandre J, Iurisci I, Grabar S, Medioni J, Ropert S. Low immunogenicity of seasonal trivalent influenza vaccine among patients receiving docetaxel for a solid tumour: Results of a prospective pilot study. Br J Cancer 2011;104:1670–4.
- Hottinger AF, George AC, Bel M, Favet L, Combescure C, Meier S, et al. A prospective study of the factors shaping antibody responses to the AS03-adjuvanted influenza A/H1N1 vaccine in cancer outpatients. Oncologist 2012;17: 436–45.
- Xu Y, Methuku N, Coimbatore P, Fitzgerald T, Huang Y, Xiao YY, et al. Immunogenicity of an inactivated monovalent 2009 influenza A (H1N1) vaccine in patients who have cancer. Oncologist 2012;17:125–34.
- Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: Preliminary report of an observer-blind, randomised trial. Vaccine 2010;28:1740–5.
- Yin JK, Khandaker G, Rashid H, Heron L, Ridda I, Booy R. Immunogenicity and safety of pandemic influenza A (H1N1) 2009 vaccine: Systematic review and meta-analysis. Influenza Other Respir Viruses 2011;5:299–305.
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G. Prevention and control of influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008;57:1–60.
- Ljungman P, Nahi H, Linde A. Vaccination of patients with haematological malignancies with one or two doses of influenza vaccine: A randomised study. Br J Haematol 2005;130:96–8.
- Vila-Córcoles A, Ochoa-Gondar O, Guzmán JA, Rodriques-Blanco T, Salsench E, Fuentes CM, et al. Effectiveness of the 23-valent polysaccharide pneumococcal vaccine against invasive pneumococcal disease in people 60 years or older. BMC Infec Dis 2010;10:73.
- Edwards JC, Szcezepanski L, Szechinski J, Filipowicz- Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004;350:2572–81.
- Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B-cells after depletion with rituximab in patient with rheumatoid arthritis. Arthritis Rheum 2006;54:613–20.
- Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol 2010; 120:76–85.
- Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: A review of infection, frequency, morbidity, mortality and vaccine responses. Lancet Infect Dis 2009;9: 493–504.
- Pauksens K. Long-term follow-up in patients with HIV vaccinated with pandemic influenza A(H1N1)/09 AS03- adjuvanted split virion vaccine and seasonal trivalent influenza split virion vaccine. Infect Ecol Epidemiol 2013;3.
- Loulergue P, Mir O, Alexandre J, Ropert S, Goldwasser F, Launay O. Low influenza vaccination rate among patients receiving chemotherapy for cancer. Ann Oncol 2008;19: 1658.

