To the Editor,
Breast cancer is the most common malignancy of women and worldwide more than 1 million new cases are diagnosed annually [Citation1]. At time of diagnosis, 10% have a metastatic disease and 20% will develop it within 10 years of their initial diagnosis [Citation2]. The goal of treatment is prolongation of life and maintenance of quality of life. Recently a new drug, eribulin, was launched in this context.
The primary objective of this retrospective study was to evaluate the effects of eribulin in breast cancer patients outside a clinical trial. These circumstances can result in treating patients with more comorbidities and large tumor burdens, because there are no well-defined exclusion criteria. This could lead to more pronounced side effects and reduced antitumor response than compared to patients included in a clinical trial as, e.g. EMBRACE [Citation3]. In this study treatment with eribulin was compared with treatment of physicians’ choice in patients with locally recurrent disease or metastatic breast cancer previously treated with 2–5 lines of chemotherapy regimens. The study demonstrated a statistically significant increase in overall survival in the eribulin-treated group (median 13.1 months) compared with treatment of physicians’ choice (median 10.6 months). In the same study eribulin had a manageable side effect profile [Citation3].
Material and methods
This study was conducted at Aarhus University Hospital and Herning Hospital recruiting patients with locally advanced breast cancer and metastatic breast cancer who had previously been treated with an anthracycline and/or a taxane.
The study included 44 patients treated with eribulin from September 2011 until August 2013. One patient was excluded due to dose delay > 2 weeks and three patients due to death from severe progression of their cancer after only one cycle of treatment.
Eribulin mesylate 1.23 mg/m2 was administered i.v. over a period of 2–5 minutes on days 1 and 8 of each 21-day cycle. In HER2-positive patients eribulin was given in combination with trastuzumab 6 mg/kg every three weeks. Treatment continued until disease progression, unacceptable toxicity or until the patient decided to withdraw from treatment. Dose reductions were permitted to manage toxicity. The day 8 dose could be omitted due to neutropenia, trombocytopenia or at the physicians’ choice.
Tumor responses in target lesions were evaluated according to Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST) [Citation4] at baseline and after every three cycles of treatment or sooner if disease progression was suspected.
After each cycle patients reported their adverse events in a systematic manner according to Common Terminology Criteria for Adverse Events (CTCAE 3.0 version).
Results
All our patients were heavily pre-treated with a median of four previous chemotherapy regimens (). The previous chemotherapy regimens, involving both adjuvant treatment and treatment for advanced breast cancer, were taxanes (97.5%), capecitabine (92.5%), anthracycline (90%), vinorelbine (67.5%) and others (CMF, gemcitabine) (17.5%). Eighteen patients showed HER2-overexpression and seven patients had triple-negative breast cancer. The most common metastatic sites were bone (72.5%), liver (60%) and lymph nodes (57.5%) and 24 patients (60%) had metastatic disease involving three or more sites (). The mean number of administrated cycles of eribulin was 4.5 cycles (range 2–12 cycles). Dose reductions and dose delays were undertaken in 27.5% and 37.5%, respectively, of the cycles provided. Median time-to-progression was 13.2 weeks. Patients showing HER2 overexpression received a mean of 4.0 cycles and patients with triple-negative breast cancer received a mean of 3.7 cycles. Objective response rate was 12.5% (ORR = CR+ PR) and clinical benefit rate was 50% (CBR = ORR+ SD).
Figure 1. Number of previous chemotherapy regimens. All our patients were heavily pre-treated with a median of four previous chemotherapy regimens.
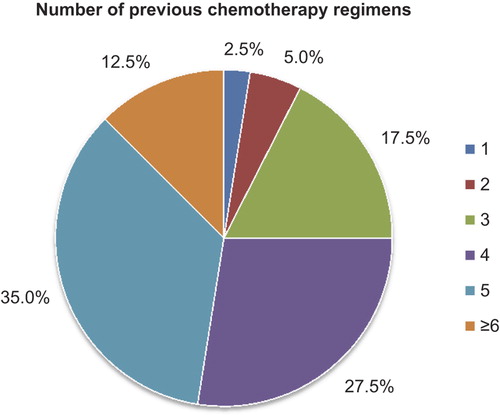
Table I. Baseline characteristics (eribulin, N = 40).
Adverse events occurred in 35 patients (87.5%). The most common adverse events were fatigue (20.3%), peripheral neuropathy (12.2%) and myalgia/arthralgia (11.4%) (). Of all reported graded adverse events 85.4% were grade 1 and 2 compared to only 14.6% reported as grade 3 and 4. Most frequent grade 3 and 4 events were fatigue, neutropenia and peripheral neuropathy.
Table II. Adverse events that occurred during treatment.
Discussion
Eribulin has been approved as treatment for patients with metastatic breast cancer, who have received at least two chemotherapeutic regimens including an anthracycline- and a taxane-based.
When comparing the effects seen in the EMBRACE study with our results, very similar effects are found, with a medium progression-free survival time of 3.7 months in the EMBRACE study and 3.4 months in our sample. Concerning the clinical benefit rate we even observed a slightly higher rate of 50% compared to the 23% reported in the EMBRACE trial.
Adverse events occurred in 99% of the patients in the EMBRACE study compared to 87.5% in our sample. The most common adverse event in the EMBRACE study as well as in our study was fatigue. In the EMBRACE study the most common grade 3 and 4 adverse event was neutropenia. We also found neutropenia to have one of the highest incidences of grade 3 and 4 adverse events along with fatigue and peripheral neuropathy. Unfortunately, no registration of the preexisting neuropathy from previous treatment with other taxane-based chemotherapy regimens was performed. In conclusion we saw a side effect profile very consistent to what we expected from prior studies.
With the effect and side effect profile of eribulin seen in this study, which is quite comparable to what is found in previous trials, it is worthwhile considering eribulin as a new standard of care. Additional considerations about when to introduce eribulin and who will benefit the most from it need to be made.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;12:69–90.
- Brewster AM, Hortobagyi GN, Broglio KR, Kau SW, Santa-Maria CA, Arun B, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst 2008;100:1179–83.
- Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE); a phase 3 open-label randomised study. Lancet 2011;377:914–23.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47.

