Abstract
Background. Currently there is no consensus on the use of adjuvant radiotherapy (RT) in retroperitoneal sarcoma (RPS). We have analysed clinical outcomes in patients with localised RPS treated at two Scandinavian Sarcoma Group (SSG) centres: Haukeland University Hospital (HUH), Bergen, Norway and Skåne University Hospital (SUH), Lund, Sweden to clarify the effects of adjuvant RT on local control and overall survival (OS).
Material and methods. Local databases and registers at HUH and SUH as well as the SSG central register were used to identify RPS patients. Patients with localised RPS who underwent surgery in Bergen between 1988 and 2009 and in Lund from 1998 to 2009 were included. Medical records were examined for clinical data, tumour characteristics, treatment factors and follow-up status. Archived tumour sections and tumour tissue were reviewed, and when necessary, restained and reclassified. Cox regression was used to analyse the association of potential prognostic factors with local recurrence-free survival (LRFS), metastasis-free survival (MFS) and OS.
Results. The study included 97 patients: 52 from Norway and 45 from Sweden. The proportion of high-grade tumours was 73%. The five-year LRFS, MFS and OS were 55%, 59% and 60%, respectively. RT was significantly associated with improved local control resulting in a five-year LRFS of 77% compared with 39% without (p < 0.001). Furthermore, five-year OS was 71% in the RT group in contrast to 52% with surgery alone (p = 0.019). In the adjusted analysis RT proved to be a significant factor also for MFS (HR = 0.42, 95% CI 0.20–0.88, p = 0.021). In addition, high-grade malignancy, large tumour and positive surgical margin were risk factors for local recurrence. High malignancy grade was the only significant adverse prognostic factor for metastasis. High age and high-grade malignancy were negative prognostic factors for OS.
Conclusion. Adjuvant RT was significantly associated with an improved five-year LRFS and OS.
Retroperitoneal sarcomas (RPS) are tumours constituting approximately 15% of all soft tissue sarcomas (STSs). Although histologically heterogeneous, these tumours are often pooled as one entity due to their retroperitoneal localisation, which has implications for the surgical approach and use of adjuvant radiotherapy (RT). The retroperitoneal space is characterised by loose tissue where tumours may grow asymptomatically and reach a considerable size before signs such as early satiety, increasing abdominal circumference, abdominal discomfort, a palpable mass or symptoms related to affected organs occur [Citation1].
Mortality has chiefly been attributed to late detection, risk of metastatic disease and local recurrence (LR). A thorough work-up with computer tomography (CT) is used to determine the site, size and proximity of the tumour to other tissues. Magnetic resonance imaging provides the best visualisation of organ involvement [Citation2]. A biopsy is usually necessary for histopathological classification of the tumour and exclusion of other diseases, and planning of appropriate treatment, i.e. if neo-adjuvant chemotherapy is indicated in chemo sensitive subtypes.
As a sole curative approach, surgery is the only definitive treatment in RPS, but controversy remains over the extent of surgery. While some centres advocate an aggressive approach with en bloc resection of uninvolved adjacent organs when feasible to secure an envelope of healthy tissue [Citation3,Citation4], other centres follow a more conservative strategy [Citation5,Citation6]. Evidence-based recommendations for routine adjuvant RT are lacking due to the paucity of randomised trials and conflicting results from retrospective studies [Citation7–10]. Neither the efficacy of RT nor the appropriate timing, whether preoperative, intra-operative or postoperative, has been established.
The Scandinavian Sarcoma Group (SSG) currently recommends participation in the European Organisation of Research and Treatment of Cancer (EORTC) randomised clinical trial investigating preoperative RT versus surgery alone for a well-defined subgroup, or, individual assessment of the indication for adjuvant RT in patients with tumours of malignancy grades 3–4 and macroscopic or microscopic positive surgical margin. The role of chemotherapy in treatment of resectable retroperitoneal sarcomas is yet to be established and is not recommended outside of clinical trials [Citation11].
The primary objective of this study was to evaluate the effect of adjuvant RT on local control and overall survival (OS). Secondly, prognostic factors for local and distant disease control were analysed.
Material and methods
A study including at least two Scandinavian sarcoma centres was necessary in order to obtain a sufficient number of patients for analysis due to the small population in Scandinavia and the rarity of RPS. Eligible patients were recruited from two well-defined regional uptake areas in Scandinavia and treated by regional multidisciplinary sarcoma teams.
RPS cases were drawn from files at Haukeland University Hospital (HUH) Bergen, Norway, and Skåne University Hospital (SUH) Lund, Sweden. The HUH cases diagnosed between 1988 and 2009 were identified from a local diagnostic database or the archive files of the Department of Pathology through appropriate morphology and localisation codes and controlled against files at the Centre for Bone and Soft Tissue Tumours, HUH.
The SUH cases diagnosed from 1998 to 2009 were identified from a local register of cases, from files of the Department of Pathology by morphology codes and from the SSG Central Register.
All cases were reviewed and reclassified by pathologists specialised in sarcoma pathology (authors) in order to validate morphological diagnoses and adjust for changes in the histopathological classifications of RPS over time. Only cases with morphologically confirmed RPS of the resected specimen were accepted [Citation12]. A total of 146 patients were identified. Of these, 97 were operated with curative intent and were included in the current analysis. Three patients were excluded from the study because their surgical treatment had been for a LR while the primary tumour had been treated prior to the inclusion period. Twenty cases were excluded because of synchronous metastasis; three because of lack of consent; and six were excluded when the revised histology showed a diagnosis other than STS. Finally, 17 did not undergo curative surgery; of these, 14 underwent debulking surgery and three were medically or technically inoperable. Data on age, sex, referral pattern, histological subtype, malignancy grade, surgery and adjuvant treatment were compiled from the medical records [Citation11]. Surgical margins were categorised as R0 (histologically negative margin), R1 (microscopically positive, but macroscopically negative margin), R2 (macroscopically positive margin) and finally RX when the surgical margin was unknown. Histological subtypes were grouped into liposarcoma, leiomyosarcoma or any other sarcoma due to the small number of other individually rare sarcoma subtypes. Malignancy grade was determined according to the four-tiered Scandinavian system and grouped into low- (SSG grade I–II) or high-grade (SSG grade III–IV), the latter corresponding to FNCLCC malignancy grade 2-3 [Citation11,Citation13]. The date of the final operation for RPS was the index event from which all time lags of clinical courses were calculated (time until verified LR, metastasis or death).
The study was performed in accordance with the World Medical Association Declaration of Helsinki, 1996, revised in 2000, and approved by the Regional Committee for Medical Research Ethics.
Statistics
Results from the two centres were compared before they were pooled. Time until LR, distant metastasis and death were the main outcome variables. Patients were stratified according to RT-status and all other parameters served as adjusting variables. Surgical margin was dichotomised into negative (R0) or positive margin (R1 + R2). Age was analysed as a continuous variable and reported as 10-year increments; similarly, tumour size was reported employing 10-cm increments.
Demographic and descriptive data were calculated as medians and ranges. χ2-tests were used to compare categorical variables and Mann-Whitney U-test was used to compare continuous variables. The Kaplan-Meier model served as univariate survival analysis in the study of the effects of RT, with the log rank (Mantel-Cox) test for comparing groups. Prognostic and treatment factors previously reported to influence survival outcomes were investigated using univariate and multivariate Cox regression analysis combined with the likelihood ratio test. Backward stepwise regression was selected to perform a limited multivariate analysis with fewer degrees of freedom and more statistical strength. A p-value of < 0.05 was considered statistically significant. IBM SPSS Statistics version 20 (SPSS Chicago, IL) was used for statistical analyses.
Results
Patient and tumour characteristics
A total of 97 patients were included: 52 from HUH diagnosed in the period 1988 to 2009, and 45 cases diagnosed from 1998 to 2009 at SUH. Patient and tumour characteristics are displayed in . When comparing the two centres, the only significant difference concerning patient and tumour characteristics was a higher frequency of high-grade malignant tumours from HUH (85% vs. 60%). The median age at diagnosis was 62 years (range 15–83), and median tumour size was 20 cm (range 4–60). Liposarcoma was the most prevalent histological type (62%) followed by leiomyosarcoma (29%). A minority of 9% constituted the category “other” including malignant peripheral nerve sheath tumour (MPNST), rhabdomyosarcoma, Ewing's sarcoma, haemangiopericytoma, angiosarcoma and synovial sarcoma. Overall 27% were classified as low-grade malignant, and 73% as high-grade malignant (). The surgical margin was negative (R0) in 54 (56%) of the surgical specimens, and positive in 37 (38%), of which 34 were R1 and three were R2. In six of the cases the surgical margin was unknown (RX).
Table I. Patient and tumour characteristics according to centre in 97 patients with RPS.
Median follow-up was 4.7 years (range 0.5–18.5) for the whole group, and 6.9 years (range 3.2–18.5) for patients still alive at final follow-up (n = 42).
Adjuvant treatment
A total of 42/97 patients (43%) underwent RT (), either preoperatively (n = 5, 12%) or postoperatively (n = 37, 88%). RT doses ranged from 20 to 65 Gy, with a median of 50 Gy. RT was administered more frequently in high-grade malignant tumours (52%) than in low-grade tumours (48%), p = 0.132.
Table II. Patient and tumour characteristics according to RT in 97 patients with RPS.
In a recording of the 26 Norwegian patients receiving RT, 15 patients (58%) had RTOG/ EORTC scale grade 1–2 nausea, one had grade 1 dysphagia and one had grade 3 nausea requiring parenteral nutritional support. Thirteen patients (50%) had grade 1–2 diarrhoea and one had grade 3 diarrhoea requiring parenteral nutritional support. Two patients had grade 2 skin toxicity, and finally, four had grade 1–2 haematologic toxicity. One patient had persistent mild diarrhoea after 90 days qualifying for late grade 1 diarrhoea. No late toxicity was reported among the Swedish patients. There were no RT-related deaths among the Norwegian or Swedish patients. Chemotherapy was only given to 15/97 (15%).
Local control
Overall, 47/97 patients experienced a LR (48%). The five-year local recurrence-free survival (LRFS) was 55%. No difference in LRFS could be demonstrated when comparing the result from the two sarcoma centres. Kaplan-Meier analysis with log rank demonstrated a statistically significant difference in five-year LRFS between the RT group (77%) and the non-RT group (39%), p < 0.001(). The five-year LRFS following negative margin was 63%, in contrast to 40% with positive margin surgery (p = 0.007). Although the quality of the surgical margin was a strong predictor for LR (), RT was significantly associated with an improved local control irrespective of surgical margin status (). RT was a significant factor also when adjusting for histotype, sex, age, size, chemotherapy, malignancy grade and surgical margin (HR = 0.20, 95% CI 0.09–0.45, p < 0.001) ().
Figure 1. (A) Local recurrence-free survival, (B) metastasis-free survival and (C) overall survival by radiotherapy in 97 patients with retroperitoneal sarcoma. With (solid line) and without radiotherapy (dotted line). Kaplan-Meier plot, p-value is from log rank test.
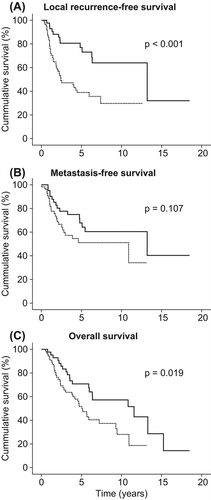
Figure 2. Local recurrence-free survival by radiotherapy and (A) negative and (B) positive surgical margin in 97 patients with retroperitoneal sarcoma. With (solid line) and without radiotherapy (dotted line). Kaplan-Meier plot, p-value is from log rank test.
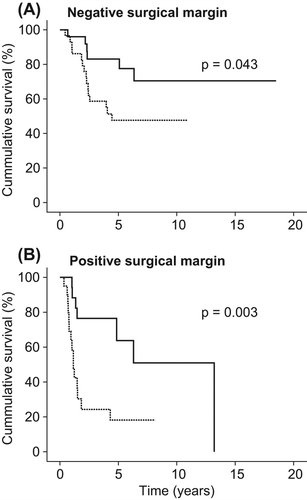
Table III. Potential prognostic factors for local recurrence by simple and multiple Cox regression analysis of 97 RPS patients.
Metastasis-free survival
Distant metastasis developed in 39/97 patients (40%). Five-year MFS for the whole group was 59%, for the RT group 68% and for the non-RT group 51% (p = 0.107) (). In the adjusted cox regression analysis there was a significant difference in MFS between the two treatment groups (± RT) (HR = 0.42, 95% CI 0.20–0.88, p = 0.021) ().
Table IV. Potential prognostic factors for metastasis-free survival by simple and multiple Cox regression analysis of 97 RPS patients.
Overall survival
A total of 55/97 (57%) patients died during the study period. Five-year overall survival (OS) for the whole group was 60%, 71% for the RT group, and finally 52% for the non-RT-group, p = 0.019 (). When adjusting for other prognostic factors, RT had a significant impact on OS (HR = 0.36, 95% CI 0.18–0.72, p = 0.004) ().
Table V. Potential prognostic factors for overall survival by simple and multiple Cox regression analysis of 97 RPS patients.
Prognostic factors
In the adjusted model, statistical significant factors for time to LR were malignancy grade, tumour size, positive tumour margin and RT (). Due to the small size of the material and restricted degrees of freedom, backward stepwise regression was performed on the four variables with the lowest p-value, confirming the significance of these factors ().
For MFS, RT and high malignancy grade were the only significant factors in multivariate analysis including backwards stepwise regression. Liposarcoma subtype was a significant favourable prognostic factor compared with leiomyosarcoma in the univariate analysis, but proved insignificant after multivariable adjustments (). High age, malignancy grade, and absence of RT had a negative effect on OS in both univariate and multivariate analyses. The same factors were significant in the backwards-stepwise regression model ().
Discussion
The most important result of this study was the significant association between RT and prolonged LRFS, MFS, and OS, and the fact that the association between RT and LRFS was evident irrespective of the surgical margin. In addition, increasing tumour size, high malignancy grade, and positive surgical margin were, as expected, associated with impaired local control. High malignancy grade was another risk factor for MFS, and high age and high malignancy grade were additional adverse factors for OS.
Our finding of RT as a significant factor for local control concurs with some [Citation9,Citation10,Citation14], but not all previous studies [Citation8]. Few other negative observational studies and no randomised trials have been published on the subject indicating both potential publication and selection bias. Unlike most studies, multivariate cox regression analysis also revealed that RT was a significant factor for both MFS [Citation15] and OS [Citation7,Citation8,Citation10,Citation15]. It is likely that LR and intraperitoneal dissemination are major reasons for disease-related death in patients with localised RPS, and that improved local control in part secured by RT could explain the favourable MFS and OS in this subset.
Eligible patients were recruited from two well-defined regional uptake areas in Scandinavia representing an average population of 2.6 million. With a relatively stable annual incidence around 2.7 cases per 106 as reported by Porter et al, this would correspond to seven new cases per year. Our findings represent 6.1 new cases per year which seems representative for the populations studied [Citation16].
The retrospective study design of this study may have resulted in heterogeneous reporting on surgical methods, resection margins as well as radiation fields due to lack of standardised registration at the time of treatment. However, treating surgeons and oncologists assisted during data accumulation to increase the quality of data.
Although consistent treatment recommendations were incomplete or absent prior to 2008, the SSG collaboration established in 1979 had led to increasing uniformity of treatment practices in the affiliated centres. Adherence to the general treatment strategies of SSG thereby supported similar, if not always identical treatment strategies in the two institutions. Indeed, there has been a shift over time towards a more aggressive surgical approach. In addition, conformal 3DRT has since the mid-1990s changed the target volume definitions and precision of RT, with further improvement of conformity with the implementation of inverse-planning techniques such as IMRT and rapid arc in the later years.
We have not analysed data concerning comorbidity, representing a potential confounding factor. However, comorbidity rarely precludes the administration of adjuvant RT if considered indicated.
Despite a uniform histopathological classification and malignancy grading system, in addition to re-evaluation of these two parameters by pathologists (authors) at the two centres, a significant higher proportion of high-grade tumours and a higher percentage of leiomyosarcoma were found at HUH [Citation11]. This finding might represent a dissimilar risk profile in the two populations. Alternatively, the practical accuracy of the SSG four-tiered grading system may be questioned. A low number of rare individual tumour types in a limited patient material, and suspected under-referral of low-grade liposarcomas to sarcoma centres due to lower morbidity and mortality, may be contributing explanatory factors. We noted a greater preponderance of high-grade malignant RPS (73%) in our material relative to previous findings [Citation4,Citation10,Citation17,Citation18]. The relative distribution of histological subtypes did not differ greatly from past studies [Citation15,Citation19]. Despite the high percentage of high-grade malignant tumours, the five-year LRFS is comparable to other studies investigating both high- and low-grade malignant tumours [Citation4,Citation20,Citation21] and intermediate to high-grade malignant RPS [Citation22,Citation23].
In the absence of conclusive clinical trials or evidence-based recommendations for adjuvant treatment, RT or chemotherapy is sometimes administered on an individual basis. Since 2008, SSG has recommended consideration of postoperative RT in high-grade malignant RPS if macroscopic tumour tissue has been left behind, or, in areas of microscopically involved tumour margin [Citation11]. Several other international guidelines support a similar approach [Citation2,Citation24]. Recently, inclusion of patients in the ongoing randomised trial of preoperative RT conducted by the EORTC has become an option at some SSG centres.
In the current study, the majority of patients underwent RT after surgery (88%) and only a small fraction received preoperative RT (12%). We did not differentiate between pre-, intra- or postoperative RT in our survival analysis due to the low number of cases and few patients receiving RT before surgery. Theoretically, preoperative RT in RPS could be beneficial as it is applied while the primary tumour is still displacing the adjacent healthy tissue beyond the radiation field. This is advantageous as it limits the radiation dose to abdominal viscera, which generally have low radiation tolerance. In Jones et al.'s study from 2012 [Citation18], preoperative external-beam RT was well tolerated as Radiation Therapy Oncology Group acute toxicity score was ≤ 2 in all patients, in contrast to toxicity scores ≥ 3 for 39% of the patients treated with postoperative RT and brachytherapy. Acute toxicity such as enteritis will be expected in most cases of RT involving the intestines and is related to total dose and volume of intestine irradiated. Very few cases of late effects have been recorded in our data set and is most likely underreported, as the retrospective design does not allow for accurate scoring of toxicity.
The typical adjuvant dose in Scandinavia is 50 Gy/25 fractions. The dose-limiting organs at risk combined with large target volumes often preclude the administration of higher doses than 50 Gy. Only four patients in the current study had doses > 50 Gy. Among the four patients that had doses < 50 Gy, one patient chose to terminate the treatment and in another patient RT was discontinued because of renal failure.
Although details on RT administration were incompletely registered, preoperative RT fields typically encompassed areas of potential intra-lesional surgery. Postoperative treatment has mainly been administered to the tumour bed in cases of microscopically positive margins, or, following R0 margins in which the uncertainty was considered substantial. The surgical margins in large multivisceral specimens are difficult to assess with certainty. When denoting an R0 margin, the resection margin was reported microscopically uninvolved. However, only a restricted area of the tumour circumference was examined by the pathologist, and we therefore believe that our frequency of R0 resections is overestimated. The margin was described as wide in only one of 54 Norwegian patients. In most cases the R0 margins were described as marginal. However, the trend towards “wide margin surgery” including adjacent organs and psoas muscle fascia might increase the likelihood of complete resections.
In this series of patients, RT was associated with a survival benefit also when adjusting for other prognostic factors, suggesting that adjuvant RT could be valuable for most RPS patients irrespective of the quality of the surgical margin. However, the seemingly positive effect of RT should not motivate less aggressive surgery to be “polished” by RT, on the contrary, the best outcome in our analysis is seen when negative margin surgery is combined with RT. This is widely accepted in extremity STS in which adjuvant RT is routinely recommended following wide margin surgery in large, high-grade, deep-seated tumours. For reasons discussed these results need confirmation in a randomised, controlled study investigating advantages and side effects of radiotherapy in both the acute phase and on long-term follow-up.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The project was supported in part by the National Competence Centre for Sarcoma, The Norwegian Cancer Society and the Department of Clinical Medicine, University of Bergen.
References
- Hueman MT, Herman JM, Ahuja N. Management of retroperitoneal sarcomas. Surg Clin North Am 2008;88:583–97.
- Schwarzbach M, Hohenberger P. Current concepts in the management of retroperitoneal soft tissue sarcoma. Recent Results Cancer Res 2009;179:301–19.
- Gronchi A, Lo Vullo S, Fiore M, Mussi C, Stacchiotti S, Collini P, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retro- peritoneal soft tissue sarcoma patients. J Clin Oncol 2009;27:24–30.
- Bonvalot S, Rivoire M, Castaing M, Stoeckle E, Le Cesne A, Blay JY, et al. Primary retroperitoneal sarcomas: A multivariate analysis of surgical factors associated with local control. J Clin Oncol 2009;27:31–7.
- Strauss DC, Hayes AJ, Thway K, Moskovic EC, Fisher C, Thomas JM. Surgical management of primary retroperitoneal sarcoma. Br J Surg 2010;97:698–706.
- Pisters PWT. Resection of some – but not all – clinically uninvolved adjacent viscera as part of surgery for retroperitoneal soft tissue sarcomas. J Clin Oncol 2009;27:6–8.
- Tseng W, Martinez SR, Tamurian RM, Borys D, Canter RJ. Histologic type predicts survival in patients with retroperitoneal soft tissue sarcoma1. J Surg Res 2012;172:123–30.
- Le Péchoux C, Musat E, Baey C, Al Mokhles H, Terrier P, Domont J, et al. Should adjuvant radiotherapy be administered in addition to front-line aggressive surgery (fas) in patients with primary retroperitoneal sarcoma?Ann Oncol 2013;24:832–7.
- Zhou Z, McDade TP, Simons JP, Ng SC, Lambert LA, Whalen GF, et al. Surgery and radiotherapy for retroperitoneal and abdominal sarcoma: Both necessary and sufficient. Arch Surg 2010;145:426–31.
- Sampath S, Hitchcock YJ, Shrieve DC, Randall RL, Schultheiss TE, Wong JYC. Radiotherapy and extent of surgical resection in retroperitoneal soft-tissue sarcoma: Multi-institutional analysis of 261 patients. J Surg Oncol 2010;101:345–50.
- Eriksson M, Joensuu H, Kristensen G, Brun E, Haugland HK, Åhlén J, et al. Recommendations for the diagnosis and treatment of intraabdominal, retroperitoneal and uterine sarcomas, ssg xvii. Lund: 2008.
- Fletcher C, Unni K, Mertens F. World Health Organization classification of tumours. Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002.
- Guillou L, Coindre J, Bonichon F, Nguyen B, Terrier P, Collin F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers sarcoma group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997;15:350–62.
- Stoeckle E, Coindre J-M, Bonvalot S, Kantor G, Terrier P, Bonichon F, et al. Prognostic factors in retroperitoneal sarcoma: A multivariate analysis of a series of 165 patients of the French Cancer Center Federation sarcoma group. Cancer 2001;92:359–68.
- Catton CN, O’Sullivan B, Kotwall C, Cummings B, Hao Y, Fornasier V. Outcome and prognosis in retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys 1994;29: 1005–10.
- Porter GA, Baxter NN, Pisters PWT. Retroperitoneal sarcoma: A population-based analysis of epidemiology, surgery, and radiotherapy. Cancer 2006;106:1610–6.
- Van De Voorde L, Delrue L, van Eijkeren M, De Meerleer G. Radiotherapy and surgery—an indispensable duo in the treatment of retroperitoneal sarcoma. Cancer 2011;117:4355–64.
- Jones JJ, O’Sullivan B, Couture J, Heisler RL, Kandel RA, Swallow CJ. Initial results of a trial of preoperative external-beam radiation therapy and postoperative brachytherapy for retroperitoneal sarcoma. Ann Surg Oncol 2002;9:346–54.
- Thomas DM, O’Sullivan B, Gronchi A. Current concepts and future perspectives in retroperitoneal soft-tissue sarcoma management. Expert Rev Anticancer Ther 2009;9:1145–57.
- Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: Analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;228: 355–65.
- Jaques DP, Coit DG, Hajdu SI, Brennan MF. Management of primary and recurrent soft-tissue sarcoma of the retroperitoneum. Ann Surg 1990;212:51–9.
- Pawlik TM, Pisters PW, Mikula L, Feig BW, Hunt KK, Cormier JN, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol 2006;13:508–17.
- Ferrario T, Karakousis CP. Retroperitoneal sarcomas: Grade and survival. Arch Surg 2003;138:248–51.
- Grimer R, Judson I, Peake D, Seddon B. Guidelines for the management of soft tissue sarcomas. Sarcoma 2010; 2010:15.

