Abstract
Background. A single-institution review of long-term outcomes and factors affecting local control (LC) following radiotherapy for non-metastatic medulloblastoma.
Material and methods. From 1963 to 2008, 50 children (median age, 7.3 years; range 1.2–18.5) with stage M0 medulloblastoma were treated with radiotherapy; half underwent a gross total resection (no visible residual tumor) or near-total resection (< 1.5 cm3 of gross disease remaining after resection). Median craniospinal dose was 28.8 Gy (range 21.8–38.4 Gy). Median total dose to the posterior fossa was 54.3 Gy (range 42.4–64.8 Gy). Eighteen patients (36%) received chemotherapy as part of multimodality management, including 11 who received concurrent chemotherapy.
Results. Median follow-up was 15.7 years (range 0.3–44.4 years) for all patients and 26.6 years (range 7.3–44.4 years) for living patients. The 10-year overall survival, cancer-specific survival, and progression-free survival rates were 65%, 65%, and 69%. The 10-year LC rate was 84% and did not significantly change across eras. Four percent of patients experienced local progression five years after treatment. On univariate analysis, chemotherapy and overall duration of radiotherapy ≤ 45 days were associated with improved LC. Patients receiving chemotherapy had a 10-year 100% LC rate versus 76% in patients not receiving chemotherapy (p = 0.0454). When overall radiotherapy treatment lasted ≤ 45 days, patients experienced a superior 95% 10-year LC rate (vs. 73% in patients treated > 45 days; p = 0.0419). Three patients (6%) died from treatment complications, including radionecrosis/cerebellar degeneration, severe cerebral edema leading to herniation, and secondary malignancy.
Conclusions. While we cannot draw definitive conclusions given the retrospective nature of our study, our long-term data suggest that reductions in craniospinal dose and boost target volume to reduce toxicity have not compromised disease control in the modern era. Our data also support analyses that implicate duration of radiotherapy, rather than interval between surgery and radiotherapy, as a factor in LC. Chemotherapy in multimodality management of medulloblastoma may have an underappreciated role in improving LC rates.
Medulloblastoma is the most common malignant solid brain tumor found in children, yet it is still rare with an incidence of 0.6 per 100,000 person years [Citation1]. The low incidence of this tumor complicates the study of effective therapeutic treatments and limits data on long-term outcomes. Medulloblastoma treatment is typically multimodal, requiring the use of surgery, adjuvant radiotherapy (RT) and chemotherapy for tumor control. The goal of RT in the management of medulloblastoma is to augment local control and prevent leptomeningeal spread [Citation2]. Today, five-year survival rates for M0 medulloblastoma patients have reached 70–87%, attributable largely to the addition of craniospinal irradiation (CSI) and chemotherapy to the historic approach of surgery alone [Citation3,Citation4]. However, it is well documented in the literature that classical high dose CSI has led to a multitude of late sequelae in survivors [Citation5]. As a result, the standard of care RT for medulloblastoma – particularly non-metastatic (M0) medulloblastoma – has been de-escalated since the 1950s, largely based on efforts to minimize radiation toxicity while maintaining adequate overall survival.
Current treatment protocols for M0 patients typically involve a radiation dose of 23.4 Gy to the neuroaxis and 54 Gy to the posterior fossa or primary tumor bed. Unfortunately, the prospective studies supporting this approach tend to focus on primary endpoints of event-free survival and overall survival. Although the efforts to reduce radiation toxicity may theoretically result in increased local recurrences, there are few studies that evaluate factors specifically impacting local control. For that reason, the aim of this study is to address that gap in the literature and evaluate patient characteristics and treatment variables associated with long-term local control.
Material and methods
Between October 1963 and December 2008, 50 patients with biopsy-proven stage M0 medulloblastoma were treated with postoperative RT at the University of Florida. Inclusion criteria included children 18 years of age or younger. Patients treated with palliative intent or for recurrent or disseminated disease (M1+) were excluded from the study. The medical records of eligible patients were retrospectively reviewed under an institutional review board-approved protocol. Patient and tumor characteristics are summarized in .
Table I. Patient, tumor, and treatment characteristics.
Treatment
All patients in this study underwent primary surgery and postoperative RT. The extent of resection was based on surgical reports, postoperative imaging, clinic notes from treating physicians, or a combination of these. Resection was categorized as subtotal when > 1.5 cm3 was left at the tumor site, near total when residual disease < 1.5 cm3 was left at the tumor site, and gross total when the tumor was completely excised. After initial surgery, 19 patients (38%) had a gross total resection (GTR), nine patients (18%) had a near-total resection (NTR), and 25 patients (50%) had a subtotal resection (STR). One patient who had an STR underwent a second-look GTR one week later.
Treatment-specific parameters are described in . The median overall RT dose was 54.3 Gy (range 42.4–64.8), with a median initial CSI dose of 28.8 Gy (range 21.8–38.4) and a median posterior fossa boost of 25 Gy (range 14.1–38.4). Dosing strategies varied considerably across treatment eras. Since 1999, the standard treatment regimen has been an initial dose of 23.4 Gy to the CSI and a posterior fossa boost of 30.6 Gy, with a total dose 54 Gy. The median interval between surgery and the RT start date was 19 days (range 5–160 days). The median duration of RT treatment was 45 days (range 30–65 days). The median interval from surgery to the end of RT was 67 days (range 41–216).
Table II. Treatment data.
Chemotherapy regimens also varied considerably across treatment eras. Early patients in the series received no chemotherapy. In total, 18 patients (36%) received chemotherapy in addition to surgery and RT. The most common chemotherapy regimens were as follows: concurrent vincristine with adjuvant vincristine, lomustine, cisplatin +/− adjuvant etoposide/cytoxan; or neoadjuvant etoposide and cisplatin +/− adjuvant vincristine/cytoxan; or concurrent vincristine with adjuvant vincristine, cytoxan, cisplatin +/− adjuvant etoposide/carboplatin. Rarely, patients received chemotherapy regimens that involved MOPP (combined mustargin, oncovin, procarbazine, and prednisone) or adriamycin.
Statistical analysis of survival was done using SAS software and the Kaplan-Meier method was used to estimate local control and survival characteristics. Durations of PFS, cause-specific survival (CSS), overall survival, local control, and time to metastases were measured from the date of first surgery to the time of event.
Results
Outcomes
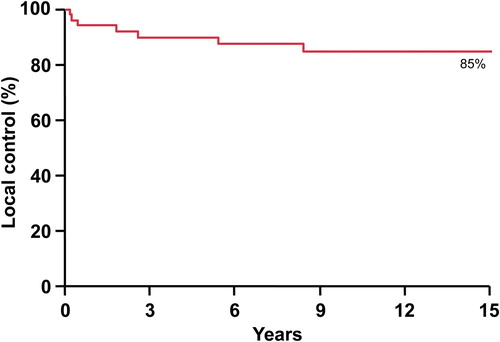
The median follow-up from diagnosis was 15.7 years (range 0.3–44.4 years). The median follow-up of surviving patients was 26.6 years (range 7.3–44.4). All patients were included in estimations of survival and disease control. Actuarial 5- and 10-year local control rates were calculated at 89% and 84%, respectively (). Actuarial 5- and 10-year progression-free survival (PFS) rates were 73% and 69%. CSS and overall survival rates were the same at 5 and 10 years at 72% and 65%.
Regarding radiation doses, 19 patients (38%) received less than 54 Gy to the posterior fossa and 31 patients (62%) received 54 Gy or greater to the posterior fossa. There was no significant correlation between the radiation dose and extent of resection. Of the patients treated, histology reports did not typically include details on anaplasia; thus, we could not use histology to stratify patients further.
Prognostic factors
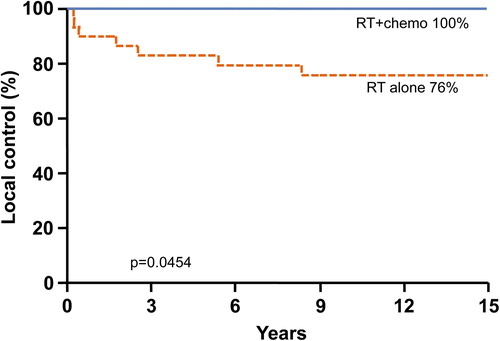
On univariate analysis improved local control correlated with patients receiving chemotherapy at any time during treatment. The local control rate at 10 years was excellent for patients who received both chemotherapy and RT at 100%. The 5- and 10-year local control rates for patients who did not receive chemotherapy were significantly lower at 84% and 76% (p = 0.0454; ). The limited number of patients made further analysis of induction versus concurrent versus maintenance chemotherapy statically invalid.
Figure 2. Kaplan-Meier estimate of local control stratified by patients who did and did not receive chemotherapy.
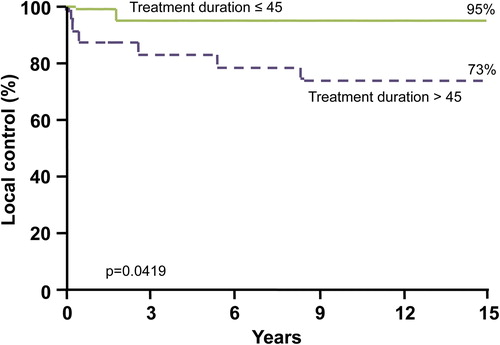
The duration of treatment also correlated with an improved local control rate. When the RT duration was less than or equal to 45 days, the 10-year local control rate was 95%. When the duration of radiation treatment exceeded 45 days, the local control rates were only 83% and 73% at 5 and 10 years (p = 0.0419; ).
Our analysis did not find any statistically significant variation in local control due to age ≤ 3 years or extent of resection. Our findings for overall survival, CSS, PFS, and local control with age stratification based on ≤ 3 years versus > 3 years yields p-values of 0.246, 0.2783, 0.4157, and 0.9895, respectively. Results from the univariate analysis also failed to show any change in local control based on treatment decades (i.e. 1960–1979, 1980–1990, and 1991–2008) or based on total radiation dose to the tumor bed. An ‘extended’ interval between surgery and initiation of RT (> 28 days) was not associated with a decreased rate of local control.
Recurrence
Patterns of progression observed in our series are presented in . Seven patients ultimately experienced a local recurrence. The median time to local progression was 1.8 years (range 0.2–8.3 years). Local recurrences occurred in three patients who had an STR and four who had a GTR. A total dose to the posterior fossa of 54 Gy or greater was delivered to three patients with a local recurrence, while four patients received less than 54 Gy (range 50–53.7 Gy). All patients with a local recurrence ultimately died of disease progression.
Table III. Patterns of progression.
Actuarial 5- and 10-year rates for freedom from metastatic progression outside of the posterior fossa were 78% and 76%. The median time to metastatic progression was 2.0 years (range 0.4–8.8 years). Ultimately, metastatic progression was seen in 11 patients (22%), six of whom had an initial STR and five of whom had a GTR or NTR. Three patients with distant metastases had also recurred locally. In patients who recurred locally and distantly, the metastases were diagnosed within five months of the local recurrence.
Four patients who developed distant metastases had received postoperative chemotherapy, while seven patients were treated with RT alone. Seven patients with distant metastases recurred within the cerebrospinal axis, either in the brain, spinal cord, or meningeal space. Three patients had metastases to the bone and one to the lymphatic system. All patients with distant metastases ultimately died of disease progression.
Toxicity
There were three episodes of grade 5 toxicity: One patient was treated with 52 Gy postoperatively and developed severe radionecrosis approximately one year after therapy, which led to death. A second patient experienced severe complications from surgery, including locked-in syndrome, and passed away from severe cerebral edema leading to herniation. Lastly, a patient developed a glioblastoma multiforme 15 years after treatment, attributed to be a secondary fatal malignancy. Other toxicities observed in this patient cohort are described in detail in a prior publication [Citation6].
Discussion
The importance of maximizing disease control during initial therapy for medulloblastoma in children is critical for patient survival. In our own series, all patients with disease recurrence died of disease; other studies also report dismal survival after relapse [Citation7,Citation8]. These findings indicate that optimization of patients’ initial treatment is critical to prevent local recurrences as well as distant spread. By analyzing factors believed to influence local control, we have identified two areas of focus that are positively associated with increased local control: the use of chemotherapy and the total time duration of the RT course.
Survival and risk stratification by clinical factors
The current study reviews patients treated as early as 1963. Since then, there have been significant improvements in both disease staging and treatment of children with medulloblastoma. Until recently, risk stratification of medulloblastoma was based on clinical features. Classic high-risk factors have included age ≤ 3 years, STR, and clinically evident metastases (stage M1 or higher) [Citation9,Citation10]. Clinically observed prognostic factors derived from studies conducted by the Children's Cancer Group (CCG-942) and International Society for Pediatric Oncology (SIOP-1) established that younger age and advanced tumor stage were associated with decreased survival [Citation10–12]. Nevertheless, there remains great heterogeneity in the literature regarding the significance of clinical prognostic factors for patients with medulloblastoma, likely because some of these studies may be underpowered to find a true difference between groups [Citation1,Citation13].
Children younger than three years old are still frequently regarded as high-risk patients. McNeil et al. conducted a Surveillance, Epidemiology, and End Results database analysis of 768 children under 20 years of age diagnosed with medulloblastoma and primitive neuroectodermal tumors and treated between 1973 and 1998 [Citation14]. They found that median survival varied by age, reporting a 39% five-year overall survival rate for children under three years old and a 55% five-year overall survival rate for children ages 4–9. This finding has been supported by another published report [Citation13]. Other studies, including our own, have failed to demonstrate age as a significant prognostic factor when all patients receive CSI and other characteristics were considered.
Another classic strategy for categorizing patients is by the extent of resection, with STR a high-risk feature. A randomized study by Zeltzer et al. comparing chemotherapy regimens among 203 patients (CCG-921) found a significant difference in the five-year PFS rate in M0 patients with GTR/NTR versus M0 patients with STR (78% ± 6% vs. 54% ± 11%) [Citation10]. A retrospective study from Children's National Medical Center found resection to strongly correlate with a difference in OS and PFS, yielding p-values of 0.0006 and 0.0001, respectively [Citation13]. On the contrary, several series as well as our findings suggest that the resection margin may not be a significant prognostic factor for local control, PFS, CSS, or OS (p > 0.8 for all measurements) if therapy is otherwise adequate [Citation8,Citation9,Citation15]. This represents an important question for future study given the incidence of surgical complication including cerebellar mutism and cranial neuropathies associated with aggressive surgical resection.
We limited our series to patients with M0 disease to isolate the group at lowest risk of metastatic relapse, thus allowing a robust examination of local control. Stavrou et al. compared survival rates for M0 and M1–4 patients and adjusted for the extent of resection and age at diagnosis. They found M stage to be an independent prognostic factor for PFS (p = 0.002) [Citation13]. Five-year estimates of PFS for patients with M0, M1, and M2 disease were reported as 70% ± 5%, 57% ± 10%, and 40% ± 8%, respectively, in the randomized trial by Zeltzer et al. [Citation10]. Most of the recurrences in patients with M+ disease were outside the posterior fossa.
While classical factors are still useful in clinical practice and have allowed for more aggressive treatment and better outcomes in ‘high risk’ patients, the classification scheme for patients with medulloblastoma continues to evolve. One important improvement includes classification of tumors by histological or molecular subtype. Classifications such as classic, desmoplastic, and anaplastic/large cell can provide prognostic information. Desmoplastic histology has the most favorable prognosis [Citation16–18], and patients with this histology might benefit from radiation dose reduction or treatment de-intensification. The rapidly emerging understanding of different molecular subgroups will further refine both our local and systemic therapies.
Disease control
Medulloblastoma is classified as a WHO grade IV tumor because of its propensity for leptomeningeal spread and local recurrence. Rates of recurrence have been reported as high as 40%, and relapses tend to follow a pattern of meningeal spread [Citation8,Citation17]. Even with the addition of RT, local recurrence may affect more patients than metastatic progression according to some series including high numbers of M0 patients [Citation15,Citation19].
Five-year local control rates for children treated with postoperative RT have been reported as high as 90% to 100% [Citation20,Citation21]. These studies have median follow-up times between 44 and 77.7 months and, thusly, might not accurately predict 10-year local control rates [Citation20–23]. Unfortunately, local recurrences can be seen after five years, as demonstrated in our study. Two of our patients recurred locally at 5.4 and 8.4 years after starting initial therapy, thereby indicating the need for long-term follow-up in these patients.
Chemotherapy typically is viewed as having a role in decreasing the rate of relapse outside of the cerebrospinal axis as well as reducing later (> 5 year) leptomeningeal relapses [Citation24]. Our study found that patients receiving chemotherapy in conjunction with RT had superior local control rates at 5 and 10 years (100%) compared to patients who did not receive chemotherapy (84% and 76%; p = 0.0454). To our knowledge, this is the first correlation regarding improved local control with chemotherapy and RT. A large prospective trial by Taylor et al. identified the use of chemotherapy with RT as a positive factor improving event-free survival in stage M0 patients over the use of RT alone [Citation15], but their study does not address local control. Most current treatment regimens involve trimodality therapy; thus, the use of these protocols should correlate with superior local control and survival rates in more recently treated patients.
While most of the clinical factors that influence prognosis are not factors that a clinician can impact (such as age of diagnosis and histologic/molecular subtype), we did find that the duration of the RT course strongly correlates with local control rates. On univariate analysis, we found that when RT treatment duration was 45 days or less, patients experienced superior local control rates. This finding is similar to the conclusion of Taylor et al. who found an improved three-year event-free survival rate of 78.5% in patients completing RT within 50 days versus just 53.7% in patients who completed RT over 50 days [Citation15]. Based on these data, we recommend protocols follow appropriate dosing guidelines for RT completion within 45 days, avoiding unnecessary gaps or delays in therapy.
Disease stratification continues to evolve and rely less on features such as the child's age at presentation; today the focus has shifted to molecular subtyping such as the WNT, SHH, Groups 3 and 4 classifications [Citation25]. As these subgroups of medulloblastoma are better understood, and identification of mutations becomes more readily available, treatment protocols will likely evolve in unison. Newer therapy plans will become targeted to a specific genetic pathway mutation, but certain clinical factors (such as length of treatment or use of adjuvant chemotherapy) impacting disease control will likely remain relevant and complement these emerging schemas.
Limitations
All retrospective studies involving rare tumors carry similar limitations. Most importantly, our findings do not prove or disprove a causal relationship between local control and various factors; rather, our findings identify hypothesis generating theories. The study size may be underpowered to find significant differences in local control for parameters such as age and degree of resection. The relatively small number of patients and events prohibit a multivariate analysis. To incorporate the largest number of patients possible for our cohort, the study population is comprised of all patients with M0 disease treated with photons at the University of Florida at the time of data analysis, including patients treated across five decades. This is both a strength and weakness. This expansive time period lends to staging and treatment heterogeneity across treatment eras. Despite this study's limitations, it provides information regarding possible modifiable treatment parameters and their interplay impacting local control in these young patients.
Conclusion
Many patient and disease characteristics that impact outcomes for medulloblastoma patients cannot be changed. Our study demonstrates two areas wherein clinicians may affect positive change in local control and survival. Chemotherapy use in conjunction with RT correlated with superior local control rates when compared to RT alone. Furthermore, local control was also improved when the total treatment duration of RT was 45 days or less. Our findings elucidate simple changes that oncologists worldwide can incorporate today in the care of children with medulloblastoma.
As prognostic variables for patients with medulloblastoma are refined, continuing efforts should be placed towards maximizing disease control while minimizing late effects, especially in low-risk patients. Low-risk patients will derive the greatest benefit from preserved neurologic function since they have the longest survival of patients diagnosed with medulloblastoma. Furthermore, improved clinical and molecular risk stratification of patients will allow for tailoring of treatment intensity.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Smee RI, Williams JR, De-Loyde KJ, Meagher NS, Cohn R. Medulloblastoma: Progress over time. J Med Imaging Radiat Oncol 2012;56:227–34.
- Fossati P, Ricardi U, Orecchia R. Pediatric medulloblastoma: Toxicity of current treatment and potential role of protontherapy. Cancer Treat Rev 2009;35:79–96.
- Frange P, Alapetite C, Gaboriaud G, Bours D, Zucker JM, Zerah M, et al. From childhood to adulthood: Long-term outcome of medulloblastoma patients. The Institut Curie experience (1980–2000). J Neurooncol 2009;95:271–9.
- Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: Results of Children’s Oncology Group trial A9961. Neuro Oncol 2013;15:97–103.
- Lackner H, Benesch M, Schagerl S, Kerbl R, Schwinger W, Urban C. Prospective evaluation of late effects after childhood cancer therapy with a follow-up over 9 years. Eur J Pediatr 2000;159:750–8.
- Christopherson KM, Rotondo RL, Bradley JA, Pincus DW, Wynn TT, Fort JA, et al. Late toxicity following craniospinal radiation for early-stage medulloblastoma. Acta Oncol 2014;53:471–80.
- Massimino M, Casanova M, Polastri D, Biassoni V, Modena P, Pecori E, et al. Relapse in medulloblastoma: What can be done after abandoning high-dose chemotherapy? A mono-institutional experience. Childs Nerv Syst Epub2013 Apr 18.
- Warmuth-Metz M, Blashofer S, von Bueren AO, von Hoff K, Bison B, Pohl F, et al. Recurrence in childhood medulloblastoma. J Neurooncol 2011;103:705–11.
- Rieken S, Mohr A, Habermehl D, Welzel T, Lindel K, Witt O, et al. Outcome and prognostic factors of radiation therapy for medulloblastoma. Int J Radiat Oncol Biol Phys 2011;81:e7–13.
- Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: Conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol 1999;17: 832–45.
- Tait DM, Thornton-Jones H, Bloom HJ, Lemerle J, Morris-Jones P. Adjuvant chemotherapy for medulloblastoma: The first multi-centre control trial of the International Society of Paediatric Oncology (SIOP I). Eur J Cancer 1990;26:464–9.
- Evans AE, Jenkin RD, Sposto R, Ortega JA, Wilson CB, Wara W, et al. The treatment of medulloblastoma. Results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg 1990;72:572–82.
- Stavrou T, Bromley CM, Nicholson HS, Byrne J, Packer RJ, Goldstein AM, et al. Prognostic factors and secondary malignancies in childhood medulloblastoma. J Pediatr Hematol Oncol 2001;23:431–6.
- McNeil DE, Cote TR, Clegg L, Rorke LB. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: A SEER update. Surveillance Epidemiology and End Results. Med Pediatr Oncol 2002; 39:190–4.
- Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 Study. J Clin Oncol 2003;21:1581–91.
- von Bueren AO, von Hoff K, Pietsch T, Gerber NU, Warmuth-Metz M, Deinlein F, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: Results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol 2011;13:669–79.
- Jones DT, Jager N, Kool M, Zichner T, Hutter B, Sultan M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature 2012;488:100–5.
- Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, et al. Medulloblastomics: The end of the beginning. Nat Rev Cancer 2012;12:818–34.
- Brasme JF, Grill J, Doz F, Lacour B, Valteau-Couanet D, Gaillard S, et al. Long time to diagnosis of medulloblastoma in children is not associated with decreased survival or with worse neurological outcome. PLoS One 2012;7:e33415.
- Paulino AC, Mazloom A, Teh BS, South M, Okcu MF, Su J, et al. Local control after craniospinal irradiation, intensity-modulated radiotherapy boost, and chemotherapy in childhood medulloblastoma. Cancer 2011;117:635–41.
- Merchant TE, Kun LE, Krasin MJ, Wallace D, Chintagumpala MM, Woo SY, et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys 2008;70:782–7.
- Kawaguchi N, Sundberg C, Kveiborg M, Moghadaszadeh B, Asmar M, Dietrich N, et al. ADAM12 induces actin cytoskeleton and extracellular matrix reorganization during early adipocyte differentiation by regulating beta1 integrin function. J Cell Sci 2003;116:3893–904.
- Carrie C, Grill J, Figarella-Branger D, Bernier V, Padovani L, Habrand JL, et al. Online quality control, hyperfractionated radiotherapy alone and reduced boost volume for standard risk medulloblastoma: Long-term results of MSFOP 98. J Clin Oncol 2009;27:1879–83.
- Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: Therapy at a crossroads. Arch Neurol 2008;65:1419–24.
- Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol 2012;123: 465–72.