To the Editor,
Neurofibromatosis type 2 is a hereditary tumor predisposition syndrome manifesting with bilateral vestibular schwannomas, schwannomas of other cranial nerves, meningiomas, spinal schwannomas, ependymomas and other gliomas. It is caused by mutations of a tumor suppressor gene called ‘Merlin’ [Citation1]. Surgery and radiation therapy are the only established treatment options [Citation2,Citation3]. Recently, a hearing improvement and a reduction of tumor volume of vestibular schwannomas and intracerebral meningiomas after systemic treatment with bevacizumab have been documented [Citation4–12]. We now report an impressive effect of bevacizumab treatment on symptomatic peripheral schwannomas with a fast and complete pain relief in a patient with neurofibromatosis type 2.
Case report
A 27-year-old woman with known neurofibromatosis type 2 was referred to our outpatient clinics because of symptomatic progression.
According to the Manchester criteria the diagnosis of neurofibromatosis type 2 was made eight years earlier, at which time the patient presented with bilateral hearing impairment. The radiological findings were consistent with the manifestation of bilateral vestibular schwannomas. In the following years both lesions were treated with stereotactic radiosurgery. Some years later, after a symptomatic progression, the right-sided tumor was treated with a partial resection. Histologically, a schwannoma (WHO grade I) was confirmed. Additionally, multiple symptomatic spinal and peripheral schwannomas were resected in the following years (Supplementary Figure 1 available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.956185).
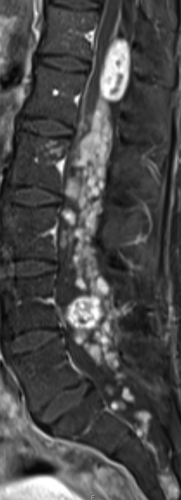
At the time of admission the patient mainly suffered from progressive left-sided hearing loss and a severe lumbovertebral pain syndrome. The brain magnetic resonance imaging (MRI) showed a progressive lesion in the left cochlear apex. shows a T1-weighted sagittal gadolinium-enhanced MRI of the lumbar spine with numerous partially confluent schwannomas. Compared to a previous MRI there was a progression of an intraspinal schwannoma at T12/L1 and a newly described schwannoma at L4 with typical features of heterogeneous contrast enhancement and some cystic changes. Due to the high number of schwannomas, no single lesion could be identified as cause for the pain.
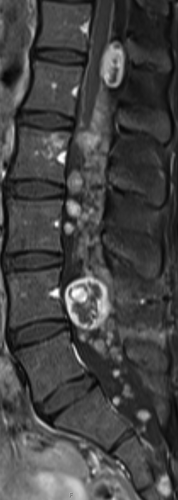
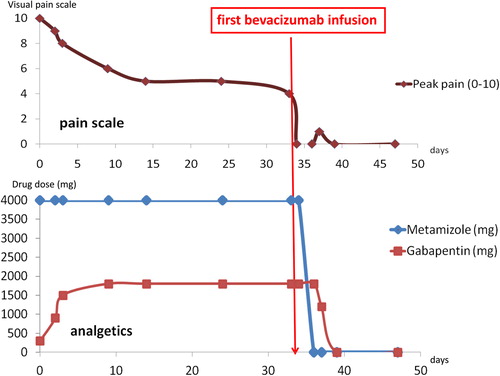
Due to the symptomatic multifocal disease progression and particularly the progressive hearing loss we decided to start an intravenous treatment with bevacizumab (5 mg/kg). Before treatment the lumbovertebral pain intensity was severely interfering with daily life even though the patient was on a combined analgetic treatment with opioids, metamizol and gabapentin. Interestingly, only a few days after the first infusion, the patient reported a dramatic reduction and finally disappearance of the lumbovertebral pain syndrome. Hence, all analgetic drugs could be stopped within two weeks. In contrast, the follow-up audiometry did not document a hearing improvement. The treatment was continued for 18 months without pain recurrence. shows MRI follow-up four months after therapy stop with mixed response, but stable disease measured by RECIST 1.1 criteria. The tumor at level T12/L1 had diminished in size, while the tumor at level L4 had increased in size and multiple additional schwannomas were stable in size.
Five months after bevacizumab was stopped the patient became symptomatic again with pain in the lumbovertebral region irradiating into the right leg. The neurological exam excluded sensomotoric deficits. New pathologic bony lesions were excluded by x-ray. We concluded that again, the known spinal schwannomas caused the pain and reinstalled a treatment with bevacizumab. Before treatment start, the patient was on an analgetic medication with metamizol and gabapentin without any relevant success; 8/10 points were documented on the visual pain scale. After applying the first infusion of bevacizumab the pain decreased again very rapidly (). The patient was pain-free without analgetic drugs two weeks thereafter.
Discussion
Neurofibromatosis type 2 is an inherited disease associated mainly with benign tumors such as schwannomas, meningiomas, ependymomas and other gliomas. A majority of these tumors can be treated with surgery or radiotherapy in the case of symptomatic disease [Citation2,Citation3]. Until recently, systemic therapy had no role in the treatment of neurofibromatosis type 2. Cytotoxic chemotherapy has no significant impact in these slowly proliferating neoplasms. Therefore, a number of potential therapeutic targets have been investigated taking into account the complex downstream signaling pathways involved in the pathogenesis of this disease [Citation4–12].
Whereas vestibular schwannomas are causing hearing deficits, pain is more often the leading symptom in peripheral schwannomas. This neuropathic pain is often difficult to manage and resistant to treatment with analgetic drugs. The patient in this case report experienced an impressive and very fast symptomatic response after only one infusion of bevacizumab.
The effect of bevacizumab on intracranial vestibular schwannomas and meningiomas has been reported before, whereas hardly any date is available on the effect on peripheral schwannomas [Citation4–12].
Antiangiogenic therapy targeting VEGF or its receptors modifies the vascular network by decreasing the number of blood vessels and normalizing the remaining vessels with regard to morphology and functionality. However, the observed effect with a pain relief within a few days most likely is not due to a reduction or normalization of blood vessels with a consecutive effect on the tumor cells. Most likely, the effect of bevacizumab is a consequence of the reduced endothelial permeability and subsequent tumor edema [Citation8]. This is supported by the fact that the mean apparent diffusion coefficient (ADC) value of the tumor at baseline is associated with radiographic response in vestibular schwannoma treated with bevacizumab [Citation9].
In summary, we document a dramatic reduction of pain caused by peripheral schwannomas after treatment with bevacizumab in a patient with neurofibromatosis type 2. These findings may warrant further investigation of bevacizumab in this specific clinical situation.
Supplementary material available online
Supplementary Figure 1 available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.956185.
ionc_a_956185_sm8805.pdf
Download PDF (1 MB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Striedinger K, VandenBerg SR, Baia GS, McDermott MW, Gutmann DH, Lal A. The neurofibromatosis 2 tumor suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP. Neoplasia 2008; 10:1204–12.
- Wentworth S, Pinn M, Bourland JD, Deguzman AF, Ekstrand K, Ellis TL, et al. Clinical experience with radiation therapy in the management of neurofibromatosis-associated central nervous system tumors. Int J Radiat Oncol Biol Phys 2009;73:208.
- Pechlivanis I, Wawrzyniak S, Engelhardt M, Schmieder K. Evidence level in the treatment of meningioma with focus on the comparison between surgery versus radiotherapy. A review. J Neurosurg Sci 2011;55:319–28.
- Plotkin SR, Stemmer-Rachamimov AO, Barker FG 2nd, Halpin C, Padera TP, Tyrrell A, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis typ 2. N Engl J Med 2009;361:358–67.
- Subbiah V, Slopis J, Hong DS, Ketonen LM, Hamilton J, McCutcheon IE, et al. Treatment of patients with advanced neurofibromatosis type 2 with novel molecularly targeted therapies: From bench to bedside. J Clin Oncol 2012; 30:e64–8.
- Goutagny S, Raymond E, Sterkers O, Colombani JM, Kalamarides M, et al. Radiographic regression of cranial meningioma in a NF2 patient treated by bevacizumab. Ann Oncol 2011;22:990–1.
- Hawasli AH, Rubin JB, Tran DD, Adkins DR, Waheed S, Hullar TE, et al. Antiangiogenic agents for nonmalignant brain tumors. J Neurol Surg B Skull Base 2013;74:136–41.
- Wong HK, Lahdenranta J, Kamoun WS, Chan AW, McClatchey AI, Plotkin SR, et al. Anti-vascular endothelial growth factor therapies as a novel therapeutic approach to treating neurofibromatosis-related tumors. Cancer Res 2010;70:3483–93.
- Plotkin SR, Merker VL, Halpin C, Jennings D, McKenna MJ, Harris GJ, et al. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: A retrospective review of 31 patients. Otol Neurotol 2012;33: 1046–52.
- Karajannis MA, Legault G, Hagiwara M, Ballas MS, Brown K, Nusbaum AO, et al. Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol 2012;14:1163–70.
- Nunes FP, Merker VL, Jennings D, Caruso PA, di Tomaso E, Muzikansky A, et al. Bevacizumab treatment for meningiomas in NF2: A retrospective analysis of 15 patients. PLoS One 2013;8:e59941.
- Mautner VF, Nguyen R, Knecht R, Bokemeyer C. Radiographic regression of vestibular schwannomas induced by bevacizumab treatment: Sustain under continuous drug application and rebound after drug discontinuation. Ann Oncol 2010;21:2294–5.