Abstract
Background. After a decade of extensive use, the actual contribution of bevacizumab in first-line treatment of metastatic colorectal cancer (mCRC) is still unclear.
Objective. To evaluate ‘real-life’ outcomes of patients with mCRC before and after the introduction of bevacizumab to standard mCRC first-line practice.
Methods. Using the computerized administrative database of Clalit Health Services’ (CHS), Israel's largest health care provider, we retrospectively compared two cohorts (n = 1739): (A) all CHS’ patients diagnosed with mCRC between January 2000 and December 2004 that received first-line irinotecan or oxaliplatin-based combination chemotherapy (before bevacizumab was introduced) (n = 1052), and (B) all patients that started first-line irinotecan or oxaliplatin combination chemotherapy together with bevacizumab between September 2006 and December 2009 (after bevacizumab was fully reimbursed in Israel for mCRC first-line therapy) (n = 687). The primary endpoint was overall survival (OS) and secondary endpoints were first-line progression-free survival (PFS) and metastatectomy rates.
Results. Median OS was longer in Cohort B than in Cohort A [23.0 months vs.15.0, adjusted hazard ratio (HR), 0.75]. Secondary outcomes were also better; PFS of 14.0 months vs. 9.8 in the earlier period (HR, 0.75) and metastatectomy rate of 8.1% versus 3.9%. The longer OS in Cohort B was preserved even after controlling for latter-line epidermal growth factor receptor (EGFR) inhibitor use (HR = 0.77).
Conclusion. In this analysis, OS, PFS and metastatectomy rates of first-line treatment of mCRC were significantly higher in the later period of the study. These results, derived from ‘real-life’ practice, suggest that the use of bevacizumab, among other alterations in the clinical management of mCRC between the two periods, might have had a significant contribution to these outcomes, and may therefore support the current practice of adding bevacizumab to first-line treatment of mCRC.
Bevacizumab (Avastin®; Genentech, Inc, South San Francisco, CA, USA), a recombinant humanized monoclonal antibody, targets the vascular endothelial growth factor (VEGF) and acts as an angiogenesis inhibitor. Bevacizumab received its initial approval by the US Food and Drug Administration (FDA) in 2004, to be prescribed in combination with 5-fluorouracil-based chemotherapy for first-line treatment of metastatic colorectal cancer (mCRC). Bevacizumab regulatory approval was based on the results of the pivotal AVF2107 phase III trial [Citation1] in which 813 previously untreated patients were randomized to bolus 5-fluorouracil (5FU)/Leucovorin (LV) and irinotecan (the IFL regimen) plus placebo or IFL plus bevacizumab. Median overall survival (OS) improved from 15.6 to 20.3 months [Hazard ratio (HR) 0.66, p < 0.001], median progression-free survival (PFS) improved from 6.2 to 10.6 months (HR 0.54, p < 0.001) and overall response rate (ORR) from 34.8% to 44.8% (p = 0.004).
By the time of regulatory approval of bevacizumab, the IFL protocol was no longer the preferred first-line backbone regimen for mCRC. Several trials have demonstrated that infusional fluoropyrimidine-based regimens with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) were more efficacious and less toxic than IFL [Citation2–4]. Whether bevacizumab provides an advantage also over the infusional regimens is still uncertain [Citation5].
The combination of bevacizumab with oxaliplatin-based chemotherapy as first-line treatment was investigated in the NO16966 randomized study of 1400 patients [Citation6]. Patients received oxaliplatin with capecitabine or 5FU/LV plus bevacizumab or placebo. Although the study was formally positive and the median PFS improved from 8.0 to 9.4 months (p = 0.0023), the results were still disappointing; neither the response rate (47% vs. 49%, p = 0.31) nor the median OS time (21.3 months vs. 19.9 months, p = 0.077) were significantly different between the groups. While several assumptions were made as to the different results in this trial compared with the pivotal study [Citation7], the NO16966 still raised significant doubts as to the real impact of bevacizumab when combined with modern regimens used in mCRC. Therefore, the purpose of this study was to retrospectively evaluate outcomes of patients with mCRC after the introduction of bevacizumab compared to outcomes before bevacizumab was available, in order to assist in resolving the concerns about bevacizumab's contribution to patients in the ‘real-life’ setting.
Reimbursement timeline in Israel
Bevacizumab was initially added to the Israeli National List of Health Services (NLHS) in April 2005 for a limited indication of first-line treatment of mCRC in which a more substantial benefit seemed to have been observed in the pivotal study: patients with primary rectal tumors or those with metastatic colon cancer who were potential candidates for hepatic metastectomy. In September 2006, the reimbursement decision was broadened to include all first-line mCRC patients and until January 2012, bevacizumab's reimbursement was restricted to first-line therapy only.
Of note, before the availability of alternative biologicals in first-line treatment of mCRC (January 2012), Israeli physicians tended to recommend bevacizumab to the vast majority of patients, excluding only those with overt contra-indications.
Oxaliplatin received reimbursement approval in January 2001, for second-line treatment of mCRC or as first-line treatment for patients who could not tolerate irinotecan. In April 2005, the reimbursement indication was broadened to include all mCRC patients. Irinotecan is reimbursed in Israel for all mCRC patients, since December 1997.
Methods
Patients diagnosed with mCRC were identified from Clalit Health Services’ (CHS) administrative database. CHS is Israel's largest healthcare organization with over four million members, serving nearly 53% of the total population, with a nationwide distribution. CHS's administrative database is an inclusive database with continuous real-time input from computerized pharmaceutical, medical, and executive operating systems.
Treatment outcomes were retrospectively compared between two cohorts, which were constructed based on the chronology of the reimbursement process of bevacizumab in Israel, as follows:
Cohort A included all CHS's patients who initiated treatment for mCRC between 1 January 2000, and 31 December 2004 that were treated with a first-line chemotherapy protocol including irinotecan or oxaliplatin. Bevacizumab was not available yet for patients in this cohort.
Cohort B included all CHS's patients that started first-line treatment for mCRC with a regimen that included irinotecan or oxaliplatin plus bevacizumab, between 1 September 2006, and 31 December 2009.
Data collected from the databases for each patient included demographic data; gender, age, Charlson co-morbidity index [Citation8,Citation9] and clinical data; chemotherapy regimens, metastatectomy surgery and date of death. Other relevant clinical information such as patient's performance status, sites of metastasis, primary tumor site, histopathological characteristics and stage at diagnosis, were not available. The cut-off date for data collection on patient treatments and outcomes for both cohorts was 30 September 2013.
Since in Cohort A's time period, oxaliplatin was reimbursed in Israel for first-line mCRC treatment, only for patients who could not tolerate irinotecan, a sub-analysis was performed to compare outcomes between the cohorts, restricting the results only to patients receiving irinotecan-based protocols in both cohorts. A second sub-analysis was performed among patients in Cohort B, to compare outcomes between oxaliplatin- and irinotecan-based regimens, to evaluate a possible interaction of benefit from bevacizumab therapy and the chemotherapy backbone.
End point definitions
The primary end point was OS and the secondary endpoints were PFS and metastatectomy rates. OS was defined as the time interval between initiation of first-line treatment for the metastatic disease until date of death or until the research cut-off date, whichever occurred first. Since we did not have access to CT imaging results, PFS was defined as the time interval between the dates of initiation of the first- and second-line therapies for the metastatic disease. We assumed this time interval as a reasonable proxy for clinical progression, since second-line treatments are usually initiated soon after a PFS event.
In patients that were not treated with a second-line protocol, it was not clear when exactly the PFS event occurred. In these patients, we used the following algorithm; if the patient died within six months after the end of first-line treatment, then we assumed that first-line treatment ended due to progression of disease and PFS event was recorded as the last day of treatment with the first-line protocol. If the patient died beyond six months after the end of first-line treatment (and no second-line treatment was given), we assumed that first-line treatment ended for reasons other than progression of disease (e.g. toxicity, ‘drug holidays’) and PFS was recorded as the date of death. In patients without a PFS event at the end of their follow-up, PFS was recorded until the date of data cut-off.
The study was approved by the Clalit Health Services’ Community Institutional Review Board (approval # 0031-13-COM).
Statistics
OS and PFS curves were generated using the Kaplan-Meier method. Multivariate Cox-regression modeling was used to adjust for the following potential confounders: age, gender, Charlson's comorbidity score, and exposure to both irinotecan and oxaliplatin. Statistical significance of comparisons of the two cohorts was determined using the t-test and the χ2-test. All statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software, version 20.0 (Chicago, IL, USA).
Results
The study population included 1739 patients: 687 in Cohort A and 1052 in Cohort B. Baseline patient demographic characteristics were similar between the two cohorts (). As expected, significantly more patients in the later cohort (Cohort B) received oxaliplatin in their first-line protocol and more were treated with an anti-epidermal growth factor receptor (EGFR) monoclonal antibody in later treatment lines.
Table I. Baseline demographics and clinical characteristics.
All efficacy endpoints were improved over time ( and ); median OS was 15.0 months (95% CI 13.4–16.6) for patients in Cohort A versus 23.0 months (95% CI 21.7–24.3) for Cohort B. The adjusted HR was 0.75 (95% CI 0.68–0.84), adjusted for bevacizumab use, age, sex, co-morbidity score and exposure to both irinotecan and oxaliplatin. Median PFS was also longer in cohort B; 14.0 months (95% CI 13.0–15.0) versus 9.8 (95% CI 9.0–10.5), adjusted HR 0.75 (95% CI 0.68–0.83). Rate of metastatectomies was higher as well in Cohort B (8.1% vs. 3.9%; p = 0.001). A further Cox regression analysis revealed that the longer OS in Cohort B was preserved even after controlling also for anti-EGFR use (HR = 0.77, 95% CI 0.69–0.86, p < 0.0001).
Figure 1. Overall survival (A) and progression-free survival (B) in patients receiving combination chemotherapy with or without bevacizumab.
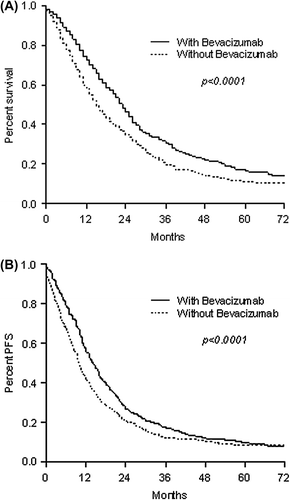
Table II. Outcomes in patients receiving combination chemotherapy with or without bevacizumab.
The sub-analysis, in which results were restricted only to patients receiving irinotecan-based first-line protocols, also demonstrated significant improvements in OS and PFS outcomes for patients in cohort B ( and ), although after controlling for anti-EGFR use, the impact on survival in these patients was not statistically significant (HR = 0.90, 95% CI 0.80–1.02, p = 0.101). In the second sub-analysis, which focused only on patients that received bevacizumab therapy (Cohort B), OS and PFS were significantly longer in patients receiving oxaliplatin-based regimens ( and ). The impact on OS was kept also after controlling for anti-EGFR use in both groups (HR = 0.74, 95% CI 0.64–0.85, p < 0.0001).
Figure 2. Overall survival (A) and progression-free survival (B) in patients receiving irinotecan-based chemotherapy with or without bevacizumab.
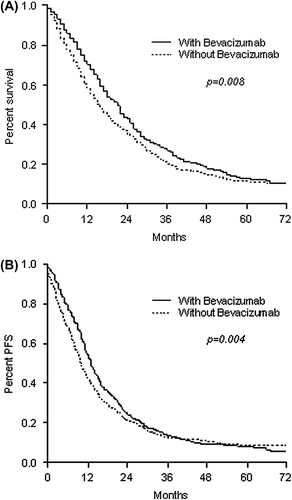
Figure 3. Overall survival (A) and progression-free survival (B) in patients receiving bevacizumab therapy with oxaliplatin/irinotecan.
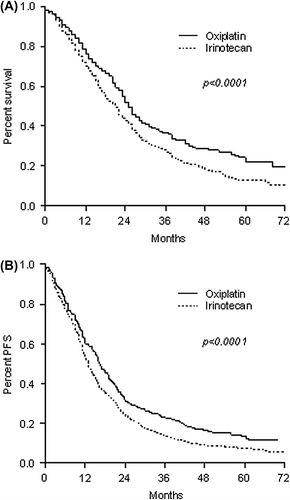
Table III. Outcomes in patients receiving irinotecan-based chemotherapy backbone.
Table IV. Bevacizumab treated patients’ outcomes, according to the chemotherapy backbone.
Discussion
This retrospective cohort study demonstrated that OS, PFS and metastatectomy rates of mCRC patients receiving first-line treatment were significantly better after the introduction of bevacizumab to standard mCRC first-line practice.
Our results in general seem to be comparable to other published retrospective evaluations of bevacizumab's use in mCRC. Meyerhardt et al. reported results of a large SEER-Medicare database bevacizumab-effectiveness analysis that included 2526 patients with mCRC diagnosed between 2002 and 2007 [Citation10]. Patients receiving bevacizumab in their first-line protocol (36% of the entire cohort) were more likely to receive oxaliplatin (82.3%) compared with patients not receiving bevacizumab (57.7%), but the distribution of tumor characteristics and patient demographics was similar. Their key finding was that, in the overall population, there was a significant benefit in OS from the addition of bevacizumab; from 15.9 months to 19.0 months (adjusted HR = 0.85). They noted that the OS advantage of bevacizumab was more apparent with irinotecan-based chemotherapy [from a median OS of 13.0 months to 18.1 months with bevacizumab (HR = 0.80, p = 0.03)] than with oxaliplatin-based chemotherapy [median OS of 19.2 months in both groups (HR = 0.96, p = 0.62)]. Due to the limitations of the SEER dataset, the authors did not report PFS data, which could have given more insight on bevacizumab's specific impact on first-line therapy of mCRC.
The BEAT [Citation11], BRiTE [Citation12] and ARIES [Citation13] studies were large, non-randomized, prospective observational cohort studies that evaluated bevacizumab in combination with different chemotherapy regimens for previously untreated patients with mCRC. In the BEAT study (1914 patients); the overall median PFS and OS were 10.8 and 22.7 months, respectively. In the parallel BRiTE study (1953 patients); PFS and OS were very similar, 9.9 and 22.9 months. Results from the ARIES study suggested that there were no significant differences in the median PFS (10.3 months vs. 10.2 months) or OS (23.7 months vs. 25.5 months) between the FOLFOX-bevacizumab (n = 968) and FOLFIRI-bevacizumab (n = 243) subgroups, respectively.
While the outcomes from the BEAT, BRiTE and ARIES studies could imply a substantial improvement over non-bevacizumab containing regimens, these cohorts did not have a control arm, and therefore firm conclusions regarding bevacizumab's potential efficacy could not be drawn.
A population-based study from the British Columbia Cancer Agency [Citation14] compared OS in 969 mCRC patients diagnosed in 2003/2004 (the ‘pre-bevacizumab era’) with 448 patients diagnosed with mCRC in 2006 (the’ bevacizumab era’). Patient demographics were similar between the cohorts. In patients that were treated with systemic therapy, usage of bevacizumab increased from 9.6% in 2003/2004 to 45.2% in 2006 (p < 0.001). Median OS increased significantly between the two time cohorts (18.6–23.6 months, p = 0.001). Since this study included patients that received bevacizumab in either first- or second-line treatment, first-line PFS was not reported and therefore bevacizumab's potential impact on OS and PFS in first-line mCRC treatment cannot be determined.
Several limitations of our current analysis must be noted. The findings of our study are restricted primarily by the nature of a retrospective database review. Imbalances between cohorts in prognostic factors that were not recorded in the CHS's administrative databases (i.e. site of primary tumor, tumor grade, surgery for primary tumor, performance status etc.) cannot be excluded. In addition, the outcome endpoints themselves have some weaknesses, as OS is not disease-specific and actual PFS data were unavailable, as described. In light of these limitations, the benefit of adding bevacizumab to first-line chemotherapy of mCRC, while suggested, cannot be determined with certainty. Moreover, it is reasonable that the benefit noted is not related only to the addition of bevacizumab; as mentioned above, in parallel to the adoption of bevacizumab, many other changes in a variety of therapeutic practice patterns in mCRC took place, such as the shift from bolus to infusional fluorouracil regimens, the higher availability of oxaliplatin in the first-line treatment, the use of EGFR inhibitors (cetuximab and later panitumumab), the use of more accurate imaging techniques (computerized tomography [CT] or positron emission tomography [PET-CT] instead of ultrasound or chest x-ray) and the more aggressive surgical management of limited stage IV disease [Citation10].
The factor with probably the largest potential influence on the results of the present study is the much more common use of anti-EGFR agents in subsequent lines in cohort B. Still, the fact that the longer OS in the later cohort was preserved in the Cox regression analysis, even after controlling for EGFR inhibitors use, together with the fact that the first-line PFS, which is unaffected by later lines of treatment, was significantly longer in cohort B, support the possible influence of bevacizumab on these outcomes.
Another potential bias on patient outcome may be related to the imbalanced use of oxaliplatin-based chemotherapy in first-line. Though, the longer OS and PFS in patients receiving oxaliplatin-based regimens might be related to the fact that at least in some Israeli centers, oxaliplatin-based first-line mCRC regimens are preferred in the perioperative setting, based on data from the EPOC trial [Citation15].This assumption is supported by other published prospective data, from registry studies such as the BEAT [Citation11], BRITE [Citation12] and ARIES [Citation13], which provide no clue to a greater impact of bevacizumab with oxaliplatin-based rather than with irinotecan-based regimens.
The actual impact of bevacizumab on resectability of liver metastases, i.e. the ability of bevacizumab to convert patients with non-resectable or borderline-resectable disease to resectable one, is currently unclear [Citation7]. Despite its limitations, our current study is encouraging as it might support an actual influence of bevacizumab on the ability to perform metastatectoimies, with more than doubling the resection rate from 3.9% to 8.1%. While such improvement can be partially explained by improved imaging methods, as well as the tendency for more aggressive resections in recent years, the magnitude of the benefit is substantial.
In spite of the described drawbacks of the present study, including the possible impact of other developments on patient outcome, our ‘real-life’ study found a higher than 50% increase in median OS, from 15 to 23 months. The median OS of patients receiving bevacizumab is strikingly within the range of other published prospective and retrospective data of bevacizumab's use for first-line treatment of mCRC [Citation7]. The internal consistency of the data, when analyzed in different ways, adds to the overall validity of the findings. Altogether, we feel that our results, derived from ‘real-life’ practice, support the potential benefit of adding bevacizumab to first-line treatment of mCRC.
Notwithstanding their limitations, observational registry or database studies are still of major importance in examining the impact of treatment in the context of real-world care settings, in populations more diverse by age, race, and health status than within a clinical trial framework. Large payer datasets, such as the CHS database, provide significant sample sizes that can produce data that assist in reflecting the actual contribution of various therapeutics, bevacizumab in first-line mCRC in this case, in the ‘real-life’ setting.
Conclusion
In this retrospective study, the results of first-line treatment of mCRC, in terms of OS, PFS and metastatectomy rates, were significantly better in the later period of the study. These results, derived from ‘real-life’ practice, suggest that the use of bevacizumab might have had a significant contribution to these outcomes, although other alterations in the clinical management of mCRC between the two research periods, such as improved imaging and more aggressive liver surgical strategies, may provide other explanations for our observation. Given that CHS covers nearly 53% of the entire Israeli population, our study can represent the general care received by Israeli patients with mCRC.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42.
- Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Results from the BICC-C Study. J Clin Oncol 2007;25:4779–86.
- Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Updated results from the BICC-C Study. J Clin Oncol 2008;26:689–90.
- Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23–30.
- Macedo LT, da Costa Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: A systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer 2012; 12:89.
- Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol 2008;26:2013–9.
- Strickler JH, Hurwitz HI. Bevacizumab-based therapies in the first-line treatment of metastatic colorectal cancer. Oncologist 2012;17:513–24.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9.
- Klabunde CN, Potosky AL, Leglar JM, Warren J. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–67.
- Meyerhardt JA, Li L, Sanoff HK, Carpenter W IV, Schrag D. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J Clin Oncol 2012;30:608–15.
- Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: The BEAT study. Ann Oncol 2009;20:1842–7.
- Kozloff M, Ulcickas Yood M, Berlin J, Flynn PJ, Kabbinavar FF, Puride DM, et al. Clinical outcomes associated with bevacizumab-containing treatment of metastatic cancer: The BRiTE observational cohort study. Oncologist 2009;14:862–70.
- Bendell JC, Bekaii-Sabb TS, Cohn AL, Hurwitz HI, Kozloff M, Tezcan H, et al. Treatment patterns and clinical outcomes in patients with metastatic colorectal cancer initially treated with FOLFOX-bevacizumab or FOLFIRI- bavacizumab: Results from ARIES, a bevacizumab observational cohort study. Oncologist 2012;17:1486–95.
- Renouf DJ, Lim HJ, Speers C, Villa D, Gill S, Blanke CD, et al. Survival for metastatic colorectal cancer in the bevacizumab era: A population-based analysis. Clin Colorectal Cancer 2011;10:97–101.
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 2008;371:1007–16.

