Abstract
Background. A large proportion of women with lymph node negative breast cancer treated with systemic adjuvant treatment do not benefit from such therapy since the patient is already cured by local treatment. Several studies have suggested that proliferation markers are strong prognostic factors in early breast cancer. Cyclins are probably the most specific markers of cell proliferation. Previously high expression of cyclin E has been associated with breast cancer recurrence.
Materials and methods. In this study we investigate the prognostic value of cyclin E1 in node negative breast cancer patients. In a population-based cohort 186 women who died from breast cancer were defined as cases and 186 women alive at the corresponding time as controls. Inclusion criteria were tumour size ≤ 50 mm, no lymph node metastases and no adjuvant chemotherapy. The study was designed to detect an odds ratio of 2.5 with a power of 90% and significance level of 0.05. Cyclin E1 was determined with immunohistochemistry (IHC) on tissue microarray (TMA).
Results. High expression of cyclin E1 was significantly associated with breast cancer death, in both uni- and multivariate analyses with odds ratios (OR) 2.3 [univariate, 95% confidence interval (CI) 1.5–3.6] and 2.1 (multivariate, 95% CI 1.2–3.5).
Discussion. Cyclin E1 is a strong prognostic factor for breast cancer death in a population-based and node negative patient cohort and can identify high-risk patients in this group.
Breast cancer is the most common cancer disease in women in the western world. The prognosis has improved significantly due to early detection and more active adjuvant treatments. About 87% of all breast cancer patients are expected to be alive five years after surgery and 78% are expected to be alive 10 years after surgery [Citation1].
Many different prognostic tools for breast cancer have been developed, most based on TNM-status, and markers of tumour biology [Citation2]. One example is The St. Gallen International Expert Consensus who recommends the use of tumour size, lymph node status, age at diagnosis, oestrogen receptor (ER) and Her2 status and Ki-67 as a base for taking therapy decision in primary breast cancer.
Until recently the use of proliferation markers as prognostic factor has been controversial mainly due to lack of consensus on methodological issues.
Immunohistochemical determination of Ki-67 is probably presently the most commonly used proliferation marker.
Ki-67 has recently being recognised by St. Gallen international expert consensus meeting and added to the general recommendations [Citation3].
Less studied markers like cyclins may have advantages over Ki-67. Unlike Ki-67 the biological function of cyclins are well known as the key proteins orchestrating the cell cycle.
Our group has recently validated Ki-67, cyclins A and B as good prognostic markers in this material taken from a population of patients with breast cancer but without lymph node metastases [Citation4,Citation5]. Both cyclins A and B seemed to be more strongly related to breast cancer outcome, than Ki-67, but the strong correlation between all these proliferation markers precluded testing the differences in strength between these markers. The present study focuses on cyclin E as a prognostic marker in the same patient material.
Cyclin E is a small protein controlling the progression and proliferation of the cell throughout the cell cycle. Cyclin E is regulating the control of G1-to S phase transition, a critical checkpoint that controls the entry into mitosis. Cyclin E itself has no enzymatic activity before it associates to cyclin-dependent kinase 2 (cdk2) in order to be activated [Citation6].
The cyclin E activated complex phosphorylates several targets that are involved in initiating DNA replication [Citation7]. Cyclin E is overexpressed in breast cancer and such overexpression is usually accompanied by the appearance of low molecular weight isoforms of cyclin E protein, which are not present in normal cells. Increased expression of cyclin E is associated with increased risk of tumour recurrence and worse outcome in several types of cancer.
In breast cancer, high expression of cyclin E has been seen related to patients with hormone receptor negative status [Citation8,Citation9] and high histological grade [Citation8,Citation10]. High expression seems to be associated to the basal-like breast cancer type [Citation11] and mutations in the BRCA1 gene [Citation12,Citation13]. In most studies high expression of cyclin E has been related to poor outcome.
Almost all previous studies were retrospective and patient materials mixed, including both node negative and positive patients with heterogeneous adjuvant therapy.
In this study we analyse the prognostic effect of cyclin E expression in a well-defined population group of breast cancer patients not treated with adjuvant chemotherapy utilising a pre-defined cut-off point.
Materials and methods
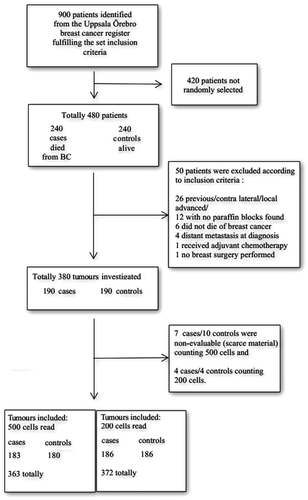
The source population of the study was a cohort of women diagnosed with breast cancer in six counties in the Uppsala-Örebro region from 1993 to 2004. Information about the patients was derived from the Uppsala-Örebro Breast Cancer Register, which is a population-based clinical database with a coverage of > 98%. Inclusion criteria were tumour size of ≤ 50 mm, no lymph node metastases, and no adjuvant chemotherapy. The number of women that met the inclusion criteria during the time period in question was 900. Within this cohort, eligible cases were defined as women who died from breast cancer. All eligible cases were selected. Women that were alive at the time of the corresponding case's death were eligible as controls.
Two hundred and forty cases were identified using the regional quality register for breast cancer and the national register for causes of death. For each identified case, one control was used. Fifty patients (10%) did not fulfil the inclusion criteria after reviewing data from patient files and pathology reports or because of missing tumour blocks: 26 patients (5.5%) had new/contralateral or locally advanced breast cancer, no paraffin blocks were found in 12 patients (2.5%), six patients (1.5%) had non-breast cancer deaths, four patients (0.8%) had distant metastases at diagnosis, one patient (0.1%) had received adjuvant chemotherapy, and one patient had no breast surgery (0.1%).
These patients and their corresponding cases/controls were not included in the study. The average age was 66 years for cases and 61 years for controls. The average tumour size was 20 mm for cases and 16 mm for controls. All patients underwent either modified radical mastectomy with axillary dissection, or conservative breast surgery with axillary dissection and postoperative irradiation of the breast. Fifty-three cases (28%) and 48 controls (25%) received endocrine therapy. Patients’ characteristics including grade, hormone receptors, and HER2 are shown in . The study was approved by the local ethics committee in Uppsala, Sweden.
Table I. Characteristics of our patients and their breast tumours.
TMA construction
Paraffin blocks from the patients’ primary tumours were collected. Haematoxylin and eosin sections were reviewed and areas with invasive tumour were selected. Each tumour was re-evaluated and reclassified according to the Elston and Ellis grading system (Amini R-M). Representative areas from each tumour were punched and brought into recipient paraffin blocks to construct TMAs consisting of two cores (diameter 1 mm) of each tumour. Then 3–4 μm thick sections were cut from array blocks and transferred to glass slides.
Immunohistochemistry
TMA slides were deparaffinised in xylene and rehydrated through a ladder of graded ethanol (absolute ethanol, 95%, 80%, and distilled water). Antigen retrieval was done in Tris-EDTA buffer (pH 9) in a microwave oven for 10 minutes (750 W) + 15 minutes (350 W) before being processed in an automatic immunohistochemistry staining machine according to standard procedures (Autostainer; Dako, Sweden). All antibodies were applied for 30 minutes at room temperature. The following monoclonal antibodies were used: Cyclin E1 (Cyclin E; HE 12, 1:200; Pharmigen, USA, mouse monoclonal). Cyclin A [Cyclin A, 1:100 NovoCastra Laboratories, UK). Cyclin B1 Dako, Sweden]. Ki67 (1:200, M7240; Dako, Sweden), ER (NCL-ER-6F11 1:150 NovoCastra Laboratories, UK) and progesterone receptor (PgR) (NCL-PGR 1:100 NovoCastra Laboratories, UK). Immunostainings were detected via DAKO Cytomation envision/HRP kit K5007. For Cyclin A, B1, E1 and Ki67 stainings, tonsil samples were used as positive controls. For ER and PgR stainings, breast cancer tissues were used as positive controls. The primary antibody was omitted from negative controls.
HER2 status was determined using immunohistochemical staining with HercepTest (DAKO). Administration definition of HER2 positivity based on the eligibility criteria for HER 2 described in our previous published studies [Citation4,Citation5,Citation14]. The immunohistochemical analyses were performed at the Department of Genetics and Pathology, Uppsala University. (Lundgren C) using a light microscope with an ocular graticule consisting of 10 × 10 = 100 grids, type Olympus biological microscope CX 31. The investigator was blinded to patient or other tumour characteristics. Hormone receptor analyses were done by a pathologist (W. Zhou). All scoring was supervised by a board-certified breast pathologist (R. M. Amini). The percentage of in cyclin E1, Ki67, cyclin A, cyclin B, ER- and PgR-positive breast cancer cells was counted in high-power fields (40 × objective) in tissue cores on TMA. Nuclear and/or cytoplasmic staining was accepted as positive reaction. When evaluating maximum scores of cyclin E1 value in percentages, we counted the high-power fields (hot spots) that had the largest proportion of positively stained cancer cells in the two biopsies and divided this by the entire number of malignant cells in these fields. If the number of cells in this high power field was insufficient the next highest field was scored, and added until a total number of 200, respectively 500 cells was counted. The percentage of positive cells was counted and calculated in approximately 200 cells (Cyclin E1200) or 500 cells (Cyclin E1500). Counting 200 cells seemed to be as accurate as counting 500 cells and was therefore chosen as the main parameter in further analysis in this study (see results).
Statistical analysis
To obtain unbiased estimates of relative risk, controls were selected by incidence density sampling, which involves matching each case to a sample of those who were at risk at the time of the case occurrence.
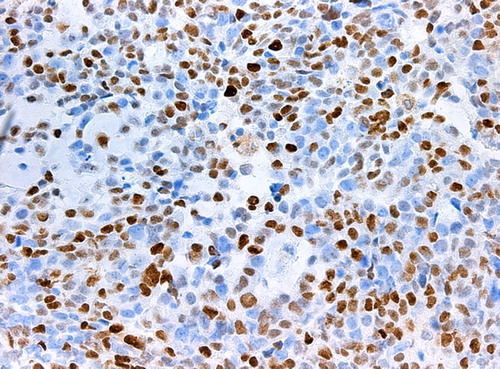
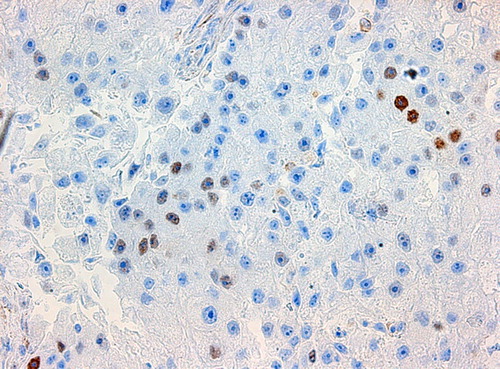
The loss in power in comparison with a complete analysis of all cohort members was small because as many women as approximately 20% of the entire cohort was chosen as controls and all eligible women with an event were studied as cases. A study based on 190 cases yields a power of 90% to detect an OR of 2.5 on a statistical significance level of 5% if we assume a prevalence of 30% of cyclin E1 over- expression in the cohort. Conditional logistic regression analysis was done to estimate ORs and confidence interval (CI) using the proportional hazard regression procedure in statistical analysis software (SAS Institute, Inc., Cary, NC, USA). Established and potential prognostic factors, such as age, tumour size, hormone receptors, histological grade, mitotic count, tubuli, nuclear atypia, Ki67, and cyclin E1 were analysed in univariate and multivariate analysis. Due to the strong correlation between cyclin E1 expression and the other cyclins, Ki-67 and grade the latter factors were excluded from the multivariate analyses, as well as PgR due to its high correlation to ER. Correlations of Ki 67, cyclin A, cyclin B and cyclin E1 to other clinicopathologic parameters were assessed with Spearman's correlation test. The cut-off values used in the study for routine stainings or clinicopathologic parameters are shown as reference values in . Cut-off values used for cyclin A, cyclin B and Ki 67 was defined as the seventh decile in line with our previous studies on cyclin A and cyclin B as well as Ki-67 [Citation4,Citation5]. This seventh decile also corresponds to the proportion of tumours with histological grade 3. For cyclin E this corresponded to 20.45% for 200 cells counted and 17.85% for 500 cells counted in this study. One example for high/ respectively low expression of cyclin E1 in IHC is shown in / respectively
Results
Correlation between cyclin E1 and other histopathological parameters
The associating of cyclin E1 expression and other tumour characteristics is shown in .
Table II. The correlation between cyclin E1 and tumour characteristics.
Cyclin E1 was correlated to histological grade (r = 0.58) and proliferation characteristics as Ki 67, cyclin A, cyclin B and mitotic count.
The correlation between cyclin E1 and Her2 tumours was weaker, but still statistically significant. Overexpression of cyclin E1 showed a significant inverse correlation to the expression of ER and PgR.
Prognostic effect of cyclin E1 expression
We first looked at the difference in the prognostic impact of cyclin E1 for breast cancer-specific survival counting either 200 cells or 500 cells in hot spots on TMAs. Evaluating 200 cells was as accurate as evaluating 500 cells. For cyclin E1200 with an OR 2.3 (95% CI 1.5–3.6) compared to cyclin E1500 OR 2.3 (95% CI 1.5–3.7) looking at the univariate model for breast cancer death. The cyclin E1 scores counting 200 cells were chosen for further evaluation in a multivariate model.
A statistically significant association was observed between breast cancer death and expression of cyclin E1, Ki 67, cyclin A, cyclin B, histological grade, mitotic count, tubuli, nuclear atypia, tumour size, ER and PgR using conditional logistic regression in a univariate model (). The prognostic value of cyclin E1 persisted in the multivariate analysis including tumour size, patient age and ER. For cases with low cyclin E1 the median time to death from breast cancer was 4.9 years while it was 3.2 years for cases with high cyclin E1. The corresponding median time to distant metastasis was three years for cases with low cyclin E1 compared to two years for cases with high cyclin. The median time from distant metastasis to death from breast cancer was 0.8 years for cases with low cyclin E1 compared to one year in cases with high cyclin E1 ().
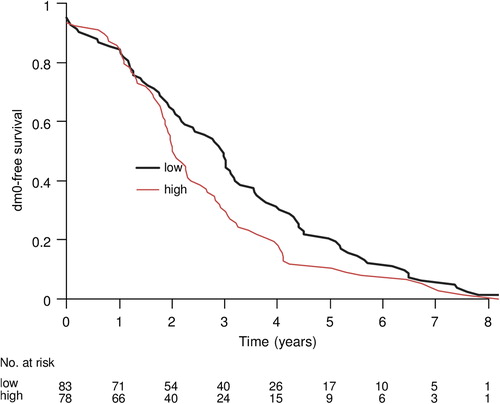
Table III. Univariate and multivariate analysis of prognostic indicators of death from low- risk breast cancer.
Discussion
This study is to our knowledge the first population based case control study designed to examine the prognostic value of cyclin E1 in a low risk node negative breast cancer population using standardised methodology regarding scoring and cut-off levels. Evaluating 200 cells concerning cyclin E1 staining in hot spots was as accurate as valuating 500 cells. Patients with high level of cyclin E1 had a 2.3 times higher risked for breast cancer-specific death. From our results cyclins, including cyclin E1, A and B, seemed to have a stronger prognostic impact on survival than the more commonly used Ki-67 (OR 1.7). The confidence levels for cyclin E1 and Ki-67 were, however, partly overlapping indicating that this difference may be due to chance; so further studies are needed to investigate whether cyclin measurement is superior to Ki-67. The prognostic impact of cyclin E1 overexpression was weaker than that of the cyclin A and B, respectively, but again, CIs were overlapping. Due to the strong correlation between the cyclins A, B, and E1, and Ki-67, our study was not powered to detect whether any of these prognostic markers are superior to others.
The significant prognostic value remained in a model adjusted for tumour size, age and endocrine therapy, indicating that this prognostic factor is giving prognostic information independently of conventional factors.
In our study Her 2 was surprisingly not a prognostic factor, probably due to the low number of patients with Her 2 overexpression (18 in cases and 13 in controls) ().
A number of individual studies and one meta-analysis [Citation7] have previously investigated the relationship between cyclin E expression and survival in breast cancer patients, while others have shown that cyclin E expression correlates to tumour size [Citation15] and stage [Citation16]. These studies were retrospective and not generally specifically designed to study patients otherwise not considered for adjuvant chemotherapy [Citation7]. Inclusion criteria, methodology and cut-off levels varied considerably. Some studies included all stages I–IV [Citation9], whereas TNM stage in others was not available [Citation12,Citation17,Citation18]. Adjuvant therapy varied and was of course dependent on tumour stage. Not surprisingly, taking into account this heterogeneity, estimates of the prognostic values differed widely in these studies.
Two previous studies in node negative breast cancer reported results similar to our study. The study by Rudolph et al., 2003, had inclusion criteria most similar to ours [Citation19]. This study, like ours, showed that cyclin E expression was significantly associated to outcome. Another study by Kuhling et al. also conducted in node negative patients found that patients having tumours with high expression of cyclin E had shorter survival [Citation20]. These results are not in line with our previously study where neither cyclin E nor abberent cyclin E was a prognostic factor in low-risk node negative breast cancer patients [Citation14]. Thus although previous studies have been small and/or heterogeneous, the present one indicate that high cyclin E1 expression is related to poor outcome in node negative breast cancer patients not treated with adjuvant chemotherapy.
Among cases, dying from breast cancer, we observed a shorter survival time in patients with high expression of cyclin E1, and this difference seemed to even more pronounce when calculating the time from diagnosis to first appearance of metastasis. This indicates that cyclin E1 expression indeed is a marker of tumour growth rate and proliferation as expected. Interestingly, no difference was noted in time between occurrence of metastasis and death. This may indicate that post-recurrence treatment may modify the prognostic effect of cyclin E1.
Previous studies indicate that benefit of chemotherapy, especially in the neoadjuvant setting, is larger in tumours with a high proliferation rate [Citation21,Citation22]. In line with this a recent study, investigation intensification of adjuvant chemotherapy by the addition of capecitabine, showed that the benefit of the intensified regimen was confined to tumours with a high proliferation rate measured by Ki-67 expression [Citation23].
The most obvious strength of our study is the design and the well-defined population based patient material focusing on patients with small node- negative tumours, where a strong prognostic factor may have the greatest impact on choice of adjuvant therapy. To avoid the biasing effect of chemotherapy, we decided not to include patients given adjuvant chemotherapy, which is a further strength of our study. The sample size (n = 372) may seem small for a prognostic study, but due to the case-controls design, the statistical power is considerably higher than a corresponding cohort study. A sample size of about 186 observed patients (we observed 186) in this study design can be seen equivalent to a cohort of approximately 1800 patients in a cohort study design. Since breast cancer death in this cohort is a quite rare event we would have to sensor during many decennia using a cohort study to have equivalent outcome comparing with this prospective case control study.
One weakness of this design is, however, that survival curves of prognostics groups cannot easily be estimated. Survival curves according to prognostic markers give information not only of differences in overall risk, but also of timing events. Proliferation markers as prognostic factors are expected to give information also of the timing of events like recurrence and death from disease, which may have clinical utility, e.g., for the planning of follow-up schedules. In this we plotted the time to events like metastatic recurrence or death for cases only (those that died). This analysis, as expected, showed that recurrence tended to occur earlier in cases with high expression of cyclin E. Interestingly, time between recurrence and death was no longer for cases with high cyclin E. One potential explanation for this may be that post-recurrence treatment, e.g., chemotherapy might modify the prognostic effect of cyclin E indicating that cases with high cyclin E expression may gain more from treatment.
A meta-analysis of publicly available microarray based gene expression studies demonstrated that gene expression assays provide similar information and the most important information they provide is the proliferation activity [Citation24]. Using proliferation expression in combination with traditionally analysed marker might give similar therapy deciding information [Citation25]. More studies are still needed in order to clarify if an optimal measurement of proliferation in addition to classical clinical variables might give prognostic and predictive information equivalent to that of the more expensive gene expression assays. The present study as well as our previous ones on cyclins as prognostic factors in early breast cancer indicate that the cyclins in addition to the more traditional Ki-67 proliferation assay are well worth investigating for putative incorporation into such a prognostic score as an alternative to gene expression assays.
In conclusion, the present study indicate that immunohistochemical assay of the cell cycle regulator cyclin E1, like cyclin A and cyclin B, previously studied by our group, are prognostic factors in early breast cancer with prognostic strength comparable to or even better than that of the proliferation antigen Ki-67.
Acknowledgements
We also want to thank Dr W. Zhou for regarding the tumours according to ER and Marit Holmqvist for supporting statistical analysis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
We are grateful to the Foundation at the Clinic of Oncology in Uppsala, Research Fund, the Regional Research fund for Uppsala/Örebro, the Swedish Cancer Society and Lion's Cancer foundation, University Hospital, Uppsala, for financial support and we wish to thank Ulrika Larsson for skilful technical assistance.
References
- Tryggvadottir L, Gislum M, Bray F, Klint A, Hakulinen T, Storm HH, et al. Trends in the survival of patients diagnosed with breast cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol 2010;49:624–31.
- Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat 1992;22:207–19.
- Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 2009;20:1319–29.
- Ahlin C, Zhou W, Holmqvist M, Holmberg L, Nilsson C, Jirstrom K, et al. Cyclin A is a proliferative marker with good prognostic value in node-negative breast cancer. Cancer Epidemiol Biomarkers Prev 2009;18:2501–6.
- Nimeus-Malmstrom E, Koliadi A, Ahlin C, Holmqvist M, Holmberg L, Amini RM, et al. Cyclin B1 is a prognostic proliferation marker with a high reproducibility in a population-based lymph node negative breast cancer cohort. Int J Cancer 2010;127:961–7.
- Weinberg RA. pRb and control of the cell cycle clock. In: The biology of cancer, 1st ed. London;Garland Science: 2006. p 255–306.
- Wang L, Shao ZM. Cyclin e expression and prognosis in breast cancer patients: A meta-analysis of published studies. Cancer Invest 2006;24:581–7.
- Bostrom P, Soderstrom M, Palokangas T, Vahlberg T, Collan Y, Carpen O, et al. Analysis of cyclins A, B1, D1 and E in breast cancer in relation to tumour grade and other prognostic factors. BMC Res Notes 2009;2:140.
- Nielsen NH, Arnerlov C, Emdin SO, Landberg G. Cyclin E overexpression, a negative prognostic factor in breast cancer with strong correlation to oestrogen receptor status. Br J Cancer 1996;74:874–80.
- Porter PL, Barlow WE, Yeh IT, Lin MG, Yuan XP, Donato E, et al. p27(Kip1) and cyclin E expression and breast cancer survival after treatment with adjuvant chemotherapy. J Natl Cancer Inst 2006;98:1723–31.
- Chappuis PO, Donato E, Goffin JR, Wong N, Begin LR, Kapusta LR, et al. Cyclin E expression in breast cancer: predicting germline BRCA1 mutations, prognosis and response to treatment. Ann Oncol 2005;16:735–42.
- Foulkes WD, Brunet JS, Stefansson IM, Straume O, Chappuis PO, Begin LR, et al. The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res 2004;64:830–5.
- Aaltonen K, Blomqvist C, Amini RM, Eerola H, Aittomaki K, Heikkila P, et al. Familial breast cancers without mutations in BRCA1 or BRCA2 have low cyclin E and high cyclin D1 in contrast to cancers in BRCA mutation carriers. Clin Cancer Res 2008;14:1976–83.
- Ahlin C, Gruhne B, Holmqvist M, Zetterberg A, Fjallskog ML. Aberrant expression of cyclin E in low-risk node negative breast cancer. Acta Oncol 2008;47:1539–45.
- Sieuwerts AM, Look MP, Meijer-van Gelder ME, Timmermans M, Trapman AM, Garcia RR, et al. Which cyclin E prevails as prognostic marker for breast cancer? Results from a retrospective study involving 635 lymph node-negative breast cancer patients. Clin Cancer Res 2006;12:3319–28.
- Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med 2002;347:1566–75.
- Bukholm IR, Bukholm G, Nesland JM. Over-expression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. Int J Cancer 2001;93:283–7.
- Lindahl T, Landberg G, Ahlgren J, Nordgren H, Norberg T, Klaar S, et al. Overexpression of cyclin E protein is associated with specific mutation types in the p53 gene and poor survival in human breast cancer. Carcinogenesis 2004;25:375–80.
- Rudolph P, Kuhling H, Alm P, Ferno M, Baldetorp B, Olsson H, et al. Differential prognostic impact of the cyclins E and B in premenopausal and postmenopausal women with lymph node-negative breast cancer. Int J Cancer 2003; 105:674–80.
- Kuhling H, Alm P, Olsson H, Ferno M, Baldetorp B, Parwaresch R, et al. Expression of cyclins E, A, and B, and prognosis in lymph node-negative breast cancer. J Pathol 2003;199:424–31.
- Poikonen P, Sjöström J, Amini RM, Villman K, Ahlgren J, Blomqvist C. Cyclin A as a marker for prognosis and chemotherapy response in advanced breast cancer. Br J Cancer 2005;93:515–9.
- Fasching PA, Heusinger K, Haeberle L, Niklos M, Hein A, Bayer CM, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 2011;11:486.
- O’Shaughnessy J, Brownstein C, Horsch S, Scotto N, Oncology US. Randomized, phase III study of adjuvant doxorubicin plus cyclophosphamide (AC), followed by docetaxel (T) with or without capecitabine (X), in high-risk early breast cancer (EBC): Efficacy results. Breast 2011; 20:S65.
- Wirapati P, Sotiriou C, Kunkel S, Farmer P, Pradervand S, Haibe-Kains B, et al. Meta-analysis of gene expression profiles in breast cancer: Toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res 2008;10:R65.
- Geradts J, Bean SM, Bentley RC, Barry WT. The oncotype DX recurrence score is correlated with a composite index including routinely reported pathobiologic features. Cancer Invest 2010;28:969–77.