Abstract
Background. Proton beam therapy (PBT) for prostate cancer generally involves the use of two lateral beams that transverse the hips. In patients with hip replacements or a previously irradiated hip, this arrangement is contraindicated. The use of non-lateral beams is possible, but not well described. Here we report a multi-institutional experience for patients treated with at least one non-lateral proton beam for prostate cancer.
Material and methods. Between 2010 and 2014, 20 patients with organ-confined prostate cancer and a history of hip prosthesis underwent proton therapy utilizing at least one anterior oblique beam (defined as between 10° and 85° from vertical) at one of three proton centers.
Results. The median follow-up was 6.4 months. No patients have developed PSA failure or distant metastases. The median planning target volume (PTV) D95 was 79.2 Gy (RBE) (range 69.7–79.9). The median rectal V70 was 9.2% (2.5–15.4). The median bladder V50, V80, and mean dose were 12.4% (3.7–27.1), 3.5 cm3 (0–7.1), and 14.9 Gy (RBE) (4.6–37.8), respectively. The median contralateral femur head V45 and max dose were 0.01 cm3 (0–16.6) and 43.7 Gy (RBE) (15.6–52.5), respectively. The incidence of acute Grade 2 urinary toxicity was 40%. There were no Grade ≥ 3 urinary toxicities. There was one patient who developed late Grade 2 rectal proctitis, with no other cases of acute or late ≥ Grade 2 gastrointestinal toxicity. Grade 2 erectile dysfunction occurred in two patients (11.1%). Mild hip pain was experienced by five patients (25%). There were no cases of hip fracture.
Conclusion. PBT for prostate cancer utilizing anterior oblique beam trajectories is feasible with favorable dosimetry and acceptable toxicity. Further follow-up is needed to assess for long-term outcomes and toxicities.
Prostate cancer is the most common non-cutaneous malignancy diagnosed in men in the US [Citation1]. For patients treated with external beam radiation therapy, conformal techniques, such as intensity modulated photon therapy, allow for dose escalation and reduction of toxicity. Proton therapy is a form of external beam radiation therapy that allows for delivery of comparably high doses to the prostate as intensity-modulated radiation therapy (IMRT) and has shown similar five-year biochemical control of > 95% for low- and intermediate-risk patients [Citation2]. Protons deliver a lower integral dose to normal tissue by virtue of the Bragg peak, a sharp rise followed by a rapid fall off in dose at the end of the beam's range. A phase III randomized trial between proton therapy and IMRT is currently ongoing.
Due to end of range uncertainties, the most common treatment approach for targeting the prostate with protons uses opposed lateral beams that traverse the hips and allow for a distal and proximal extension of the prescribed range. This arrangement also ensures that the anterior aspect of the rectum is within the lateral penumbra of the proton beam, and not the distal edge of the Bragg peak. In patients that have undergone hip replacements, this beam approach is not possible, as the beam path must avoid the prosthetic material. In such cases, anterior-oriented beams can be used. While investigators have demonstrated the dosimetric advantages of this beam approach [Citation3], the feasibility, efficacy, and early toxicity of the clinical use of anterior beams is not well described. The purpose of the current study is to review a multi-institutional experience using anterior-oriented beams for patients undergoing proton therapy for localized prostate cancer.
Material and methods
Patients
Between 2010 and 2014, a total of 20 patients with non-metastatic prostate cancer and a history of hip replacement were treated with definitive proton therapy delivered to the intact prostate using at least one anterior-oriented beam at three centers (CDH Proton Center, Warrenville, IL, USA; ProCure Proton Therapy Center, Somerset, NJ, USA; ProCure Proton Therapy Center, Oklahoma City, OK, USA). All patients were entered into a database, and patient and treatment characteristics, toxicity and follow-up data was prospectively collected. Patient characteristics are summarized in .
Table I. Patient characteristics.
Radiation therapy
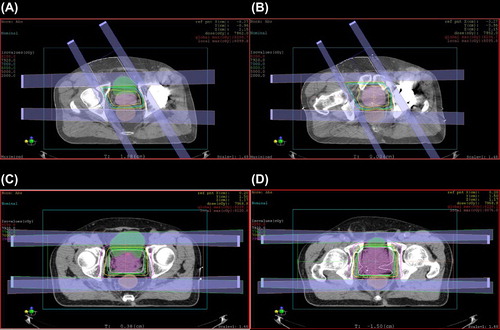
Prior to treatment, all patients had fiducial markers placed within the prostate under ultrasound guidance. Radiation therapy was delivered using uniform scanning proton beams with beam specific apertures and compensators used for each patient. Anterior-oriented beam angles that avoided the prosthetic hip, allowed for optimal target coverage, and did not exceed normal tissue constraints were selected in a forward planning fashion. Anterior-oriented beam angles ranged from 10° to 85° from vertical, from either the ipsilateral or the contralateral side of the hip replacement depending on individual patient geometry and anatomy. In the majority of cases, the anterior-oriented beam was angled 30° from vertical because most patients were treated in an inclined beam line treatment room (which has two gantry positions: lateral and 30° from vertical). For the small number of patients that were treated on a full gantry, beams that minimized internal overlap with the lateral beam and optimized sparing of internal organs were selected. Artifacts from hip replacements were addressed on a per patient basis with manual electron density override. Briefly, treatment planners contoured in fat, muscle, and bone structures in the area of the artifact. Nearby normal tissue was sampled for electron density, and the planner-generated normal tissue contours were assigned the appropriate electron density to counteract artifact effects. Beam weights of the anterior-oriented portal ranged from 12.5% to 50% of the total delivered dose, with a median of 27.5%. There were 18 patients that had corresponding lateral beams that delivered 25% to 75% of the total dose. There were two patients who were treated with anterior-oriented beams alone, without the use of a lateral beam. shows the dose distribution of a patient treated with an anterior-oriented beam from the unaffected side.
In general, 79.2 Gy [relative biological effectiveness (RBE)] was prescribed to the planning target volume (PTV) and was delivered with 1.8 Gy (RBE) delivered once daily, five times per week. The typical CTV was defined as the prostate (including the proximal seminal vesicles for intermediate- and high-risk patients). No patients were treated to the lymph nodes. The typical PTV was defined as variable expansion of the clinical target volume (CTV) (for low, 2 mm posterior and 3 mm elsewhere; for and intermediate- and high-risk patients, 3 mm posterior and 4 mm elsewhere). For lateral beams, the PTV was further expanded by 5 mm distally and proximally to account for range uncertainty, and a smear radius of 1.2 cm was applied. For anterior oriented beams, no PTV expansion was used in the distal/posterior direction. The plan was generated to cover the CTV and evaluated for range uncertainty of + / − (2.5% + 2mm). A smear radius of 0.8 cm, equal to the lateral setup uncertainty, was also employed.
Patients were immobilized and treated with a full bladder in the supine position using a custom immobilization device. Prior to treatment, a contrast-filled rectal balloon was inserted into the rectum. Daily portal films utilizing orthogonal KV x-rays taken at 0° and 90° were used for setup and position correction. At 0°, the previously placed fiducial markers were clearly seen and used to align patients in the superior inferior and left-right directions. For the lateral film, the anterior wall of the contrast-filled rectal balloon was used to align the patient in the anterior-posterior direction. The patients were then treated with this balloon in place. Three patients (15%) were also treated with concurrent androgen deprivation therapy. At Procure New Jersey, we developed an in vivo dose verification system that has been described elsewhere [Citation4]. Briefly, an endorectal balloon was circumferentially loaded with six thermoluminescent dosimeters and encased in a condom and used for the initial patient treated with an anterior-oriented beam. The balloon was inserted into the rectum and inflated with saline. In vivo doses were collected daily for three fractions for this patient.
Follow-up
Prior to treatment, baseline urinary, bowel and sexual profiles and a baseline International Prostate Symptom Score (IPSS) were collected. While on treatment, patients were assessed weekly. In general, patients were seen every three months after treatment during the first year. Gastrointestinal and genitourinary toxicity was assessed per the Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Grade 1 toxicity was defined as minimal side effects not affecting activities of daily living (ADL), grade 2 was defined as requiring initiation or increase in medication for symptom management, grade 3 was defined as severe non-life threatening toxicities or toxicities necessitating medical procedures, and grade 4 was defined as life threatening. Erectile dysfunction was defined as a change from baseline in the ability to achieve erections suitable for intercourse. Grade 1 was minimal dysfunction and Grade 2 was moderate dysfunction, requiring the initiation or increase in medication. Toxicities were considered acute if occurring during treatment and within the first 90 days after treatment.
Control Cohort
In order to compare dosimetry to a lateral beam approach, we analyzed a cohort of five patients without hip replacements that were treated with opposed lateral beams to the prostate alone consecutively at a single center. Dosimetric parameters were obtained through chart review and tabulated.
Results
The median follow-up among all patients is 6.4 months. To date there have been no biochemical or distant relapses.
The results of the in vivo dosimetry measurements from the initial patient treated with anterior-oriented beams showed agreement to within 4% of the doses predicted by the treatment planning system within the high dose region [Citation4]. This method provided a feasible approach to verify in vivo dosimetry with use of non-standard beams. Adequate target coverage was achieved for all patients, with a median PTV D95 of 79.2 Gy (RBE) (range: 69.7–79.9) and PTV V98 of 99.9% (range 98.5–100). The median PTV size was 98.0 cm3 (range 64.2–182.0). The median rectal V70 was 9.2% (range 2.5–15.4). The contralateral femoral heads were kept below tolerances in most patients. The median V45 was 0.01 cm3 (range 0–16.6) with a median maximum point dose of 43.7 Gy (RBE) (range 15.6–52.5).
When compared to the cohort of patients treated with lateral beams, both low and high dose parameters to the bladder were increased with the use of anterior oriented beams. The median bladder V50 was 12.4% (range 3.7–27.1) and the bladder V80 was 3.5 cm3 (range 0–7.1). The mean bladder dose ranged from 4.6 to 37.8 Gy (RBE) with a median of 14.9 Gy (RBE).
When compared to the cohort of patients treated with lateral beams, anterior oblique beams non-significantly increased dose to the rectum (rectal V70 9.2% vs. 6.8%, p = 0.25) and femoral head max point dose [43.7 Gy (RBE) vs. 36.8 Gy (RBE) p = 0.50]. Interestingly, bladder V80 was significantly lower (3.5 cm3 vs. 5.4 cm3, p = 0.02) and V50 and mean bladder dose were insignificantly lower (12.4% vs. 15.6%, p = 0.99 and 14.9 Gy vs. 15.2 Gy, p = 0.42, respectively) with the use of anterior-oriented beams in our cohort. This may be due to the fact that although the anterior beam is partially coursing through the bladder, the weight of this beam is decreased compared to the equal weighting typically given to two lateral beams.
Target coverage and doses to normal tissue for the cohort treated with lateral beams and the cohort treated with anterior oriented beams are summarized in . An example dose-volume histogram of a patient treated with an anterior-oriented beam (a) and lateral beams (b) is shown in .
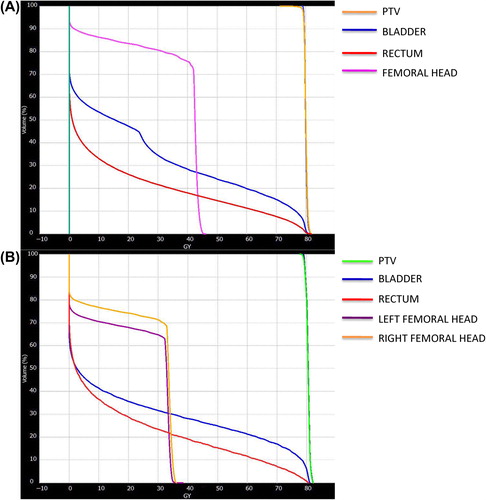
Table II. Median target coverage and dose to OARs.
Treatment was generally well tolerated. No patients required a treatment break. The most common side effect was Grade 1 dermatitis in 18 patients (90%). There were nine (45%) and eight (40%) patients with acute Grade 1 and Grade 2 acute urinary toxicities, respectively. There were five patients with Grade 1 acute bowel toxicities (25%) and no patients with Grade 2 acute bowel toxicities. There was one patient (5%) that experienced erectile dysfunction requiring the use of medication in the acute setting. There were no Grade ≥ 3 acute toxicities.
There was one patient who developed late Grade 2 proctitis complicated by the development of an anorectal ulcer on the anterior wall of the rectum (in the high dose region). He was managed with topical steroids with eventual improvement of his symptoms.
There were five patients (25%) that experienced Grade 1 hip pain. There were no further hip toxicities. Toxicity data is reported in .
Table III. Toxicity.
Discussion
In our study, definitive proton therapy to the intact prostate utilizing at least one anterior-oriented beam proved to be dosimetrically feasible and well tolerated, with no biochemical or distant failures to date and an acceptably low toxicity profile.
Proton therapy offers the unique treatment advantage of being able to deposit relatively low amounts of dose until particles come to the end of their range, at which point a large amount of energy is maximally deposited and then falls to nearly zero. These properties offer the ability to deliver high doses to the target, with minimal amounts of dose deposited beyond the prescribed range. The largest series of proton therapy used in prostate cancer involved 1255 patients treated at Loma Linda University Medical Center. Proton therapy yielded disease-free survival rates comparable to other forms of local therapy. Freedom from Grade 3 and 4 GI and GU toxicity at 5- and 10-years were 99%, respectively [Citation5]. Several other series have confirmed the effectiveness and tolerability of proton therapy in the treatment of prostate cancer [Citation2,Citation6–8]. A recent publication also showed lower rectal urgency and bowel movement frequency with proton treatment compared to IMRT [Citation9].
In current clinical practice, most patients are treated with opposed lateral proton beams. Investigators have shown that anterior-oriented beam arrangements can decrease high dose levels to the rectal wall, penile bulb and femoral heads [Citation3], however, this has not been put into routine clinical use because of several potential pitfalls of anterior beams. First, the prescribed range of protons directed to the prostate includes an additional percentage of treatment depth beyond the target volume in order to account for range uncertainty and the associated risk of underdosing the target. As the prostate is a relatively deep-seated target, prostate treatment is even more susceptible to range uncertainty than more superficial targets, and an even greater range extension is needed. An anterior beam arrangement causes this extension to fall into the rectum or bladder, whereas parallel-opposed lateral fields ensures that the extra prescribed range falls into tissues of the lateral pelvis. Secondly, the true RBE value of the distal edge of the Bragg Peak is potentially much higher than the conventional value of 1.1 [Citation10,Citation11]. Anterior-oriented beams would place this region of biological uncertainty into sensitive structures, such as the rectum and bladder. The use of lateral beams allows the distal edge of the beam and its high RBE to fall outside of these radiosensitive structures. Finally, anterior beams must travel through the bladder and are susceptible to changes in bladder and rectal filling. For example, Thörnqvist et al. recently demonstrated substantial inter-fraction variations in patients treated with proton therapy for prostate cancer that was thought to be driven by variable rectal volume [Citation12]. Lateral beams are more robust as they travel through relatively fixed tissues.
In certain clinical situations, including patients with prosthetic hip replacements, lateral fields are not an option. The dosimetric uncertainty of the prosthetic material or the risk of toxicity when treating through a previously treated hip requires that the hip be avoided. Several studies have investigated the dosimetry and technique of photon therapy for prostate cancer patients with hip replacements [Citation13–20]. Recently, investigators from Oklahoma City reported a study that compared proton therapy to volumetric arc therapy in hip replacement patients [Citation21]. However, the experience in using protons for patients with hip prosthesis remains limited, and due to the aforementioned issues with anterior beams, many institutions have chosen not to treat these patients with proton therapy and instead recommend other treatment options.
To further describe the feasibility and safety of the use of anterior oblique portals at our institutions, we analyzed a multi-institutional cohort of 20 patients treated with at least one anterior-oriented proton beam for organ confined prostate cancer, representing the largest cohort to date treated with this beam orientation. In general, all pre-defined organ constraints used for treating with lateral beams were achieved among the patients treated with anterior beams. The incidence of Grade 2+ acute urinary toxicity is higher in the current study than in some series [Citation22] but comparable to other series [Citation8]. Possible explanations include the fact that Grade 2 toxicities in the current study were exclusively initiation or increases in any medication including over the counter medications, whereas in other series, these data were tabulated but not counted as Grade 2 toxicities. It is also possible that a yet undefined dosimetric parameter is contributing to acute bladder toxicity and is increased compared to a lateral beam approach. Close follow up is planned to determine the incidence of late urinary toxicity. However, the absence of Grade 3 urinary toxicities in the current cohort is encouraging.
The anterior-oriented beams had at least part of the distal edge ranging into the rectum. Uncertainties regarding the end of range and the RBE are valid sources of concern for increased rectal toxicity. To mitigate this uncertainty, the lateral beams in the current study were weighted such that approximately 25% of the dose was delivered by the anterior- oriented beam. This is consistent with our practice in other disease sites, where up to one third of the dose can be ranged out toward critical structures if needed. We did not observe excessive acute rectal toxicity, suggesting that although these uncertainties exist, they are not clinically significant as long as the majority of the dose does not range out into a critical structure. However, close follow-up is necessary to investigate any potential chronic adverse events.
Another potential source of toxicity is the lateral beam through the contralateral, intact hip. Proton therapy utilizing non-pencil beam scanning opposed lateral beams increases dose to the femoral necks compared to photon therapy. The speculation that patients may be at risk of hip fracture and hip pain after proton therapy has been previously studied [Citation23], even though patients were not found to be at risk for hip fracture in that study. When an anterior beam arrangement is used, planners will often plan for maximal delivery through the single lateral beam in order to minimize the amount of dose coming from the anterior-oriented beams. The heavily treated contralateral hip may be at risk for dermatitis, hip pain and hip fracture. In the current study, we did not observe an excess of either skin or hip toxicities, even though as much as 75% of the total dose was delivered through the lateral beam. Notably, the incidence of patient reported hip pain was slightly higher in the current study than reported by Valery et al. (25% vs. 16%). This suggests that as long as femoral head dose constraints are observed, the risk of toxicity to the hip and overlying skin is low. Moreover, all patients were treated with uniform scanning. We would expect even lower hip and skin doses with pencil beam scanning since this will allow for proximal beam modulation.
Although patients were followed in a prospective fashion, the findings are limited by the retrospective design. Treatment approaches were non-standardized and heterogenous between institutions, limiting the ability to make specific recommendations for the use of specific beam angles. Finally, longer follow-up is needed to further characterize the toxicity and oncologic outcomes of patients. Additional follow-up is planned to confirm the findings of the study. Despite these limitations, the findings of this study provide compelling evidence of the safety and efficacy of using anterior-oriented beams in patients with a contraindication to an opposed lateral arrangement.
This evaluation is particularly timely. Statistical projections have estimated the demand for hip replacements in the US to increase by 174% by the year 2030 [Citation24], and suggest that patients with hip replacements requiring treatment for prostate cancer will be an increasingly common clinical dilemma. Moreover, there is increasing interest from some proton centers using this non-standard beam orientation in the elective setting as a way to improve conformality by taking advantage of the distal dose fall-off for patients without a contraindication. The advent of pencil-beam scanning will allow for more conformal dose distributions and may provide decreased dose to normal tissue with an anterior approach. Ongoing efforts to better understand the radiobiological properties of the distal end of the Bragg peak will also serve to lessen uncertainty and allow for providers to take better advantage of this highly advantageous portion of the proton beam—a strategy that will almost certainly rely on anteriorly directed beams.
In this largest cohort to date of patients treated with anterior-oriented proton beams for non-metastatic prostate cancer, adequate target coverage was achieved and treatment was extremely well tolerated with an acceptably low level of toxicity. Further follow-up is needed to confirm the long-term durability of this treatment and low rates of late toxicity. Further study is needed to help characterize the role for anterior-oriented beams and which patients may benefit from this approach, as conformal dose delivery continues to advance and end of range uncertainties are reduced.
Declaration of interest: Drs. Chon, Tsai and Cahlon have minority investment in ProCure Proton Therapy Center, New Jersey.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30.
- Mendenhall NP, Hoppe BS, Nichols RC, Mendenhall WM, Morris CG, Li Z, et al. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014;88:596–602.
- Tang S, Both S, Bentefour H, Paly JJ, Tochner Z, Efstathiou J, et al. Improvement of prostate treatment by anterior proton fields. Int J Radiat Oncol Biol Phys 2012;83:408–18.
- Mah D, Chen C, Cahlon O, Tsai H, Hug E, Hsi W, et al. A method for in vivo intracavitary surface dose validation using thermoluminescent dosimeters (TLDS) applied to prostate cancer patients treated with protons. Int J Radiat Oncol Biol Phys 2013;87:S741–S2.
- Slater JD, Rossi CJ, Jr., Yonemoto LT, Bush DA, Jabola BR, Levy RP, et al. Proton therapy for prostate cancer: The initial Loma Linda University experience. Int J Radiat Oncol Biol Phys 2004;59:348–52.
- Mayahara H, Murakami M, Kagawa K, Kawaguchi A, Oda Y, Miyawaki D, et al. Acute morbidity of proton therapy for prostate cancer: The Hyogo Ion Beam Medical Center experience. Int J Radiat Oncol Biol Phys 2007; 69:434–43.
- Nihei K, Ogino T, Onozawa M, Murayama S, Fuji H, Murakami M, et al. Multi-institutional Phase II study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. Int J Radiat Oncol Biol Phys 2011;81:390–6.
- Mendenhall NP, Li Z, Hoppe BS, Marcus RB, Jr., Mendenhall WM, Nichols RC, et al. Early outcomes from three prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:213–21.
- Hoppe BS, Michalski JM, Mendenhall NP, Morris CG, Henderson RH, Nichols RC, et al. Comparative effectiveness study of patient-reported outcomes after proton therapy or intensity-modulated radiotherapy for prostate cancer. Cancer 2014;120:1076–82.
- Robertson JB, Williams JR, Schmidt RA, Little JB, Flynn DF, Suit HD. Radiobiological studies of a high-energy modulated proton beam utilizing cultured mammalian cells. Cancer 1975;35:1664–77.
- Britten RA, Nazaryan V, Davis LK, Klein SB, Nichiporov D, Mendonca MS, et al. Variations in the RBE for cell killing along the depth-dose profile of a modulated proton therapy beam. Radiat Res 2013;179:21–8.
- Thörnqvist S, Muren LP, Bentzen L, Hysing LB, Hoyer M, Grau C, et al. Degradation of target coverage due to inter-fraction motion during intensity-modulated proton therapy of prostate and elective targets. Acta Oncol 2013;52:521–7.
- Kung JH, Reft H, Jackson W, Abdalla I. Intensity-modulated radiotherapy for a prostate patient with a metal prosthesis. Med Dosim 2001;26:305–8.
- Su A, Reft C, Rash C, Price J, Jani AB. A case study of radiotherapy planning for a bilateral metal hip prosthesis prostate cancer patient. Med Dosim 2005;30:169–75.
- Brooks C, Cheung RM, Kudchadker RJ. Intensity- modulated radiation therapy with noncoplanar beams for treatment of prostate cancer in patients with bilateral hip prosthesis-a case study. Med Dosim 2010;35:87–91.
- Fattahi S, Ostapiak OZ. An opposed matched field IMRT technique for prostate cancer patients with bilateral prosthetic hips. J Appl Clin Med Phys 2012;13:3347.
- Martin DA, Hruby G, Whitaker MK, Foo KY. Constrained-beam inverse planning for intensity-modulated radiation therapy of prostate cancer patients with bilateral hip prostheses. J Med Imaging Radiat Oncol 2012;56:703–7.
- van der Est H, Prins P, Heijmen BJ, Dirkx ML. Intensity modulated radiation therapy planning for patients with a metal hip prosthesis based on class solutions. Pract Radiat Oncol 2012;2:35–40.
- Kling J, Patel KM. Prostate treatment with helical TomoTherapy in patients with bilateral hip prostheses – two case studies. Med Dosim 2013;38:30–4.
- Voet PW, Dirkx ML, Breedveld S, Heijmen BJ. Automated generation of IMRT treatment plans for prostate cancer patients with metal hip prostheses: Comparison of different planning strategies. Med Phys 2013;40:071704.
- Rana S, Cheng C, Zheng Y, Hsi W, Zeidan O, Schreuder N, et al. Dosimetric study of uniform scanning proton therapy planning for prostate cancer patients with a metal hip prosthesis, and comparison with volumetric-modulated arc therapy. J Appl Clin Med Phys 2014;15:4611.
- Henderson RH, Hoppe BS, Marcus RB, Jr., Mendenhall WM, Nichols RC, Li Z, et al. Urinary functional outcomes and toxicity five years after proton therapy for low- and intermediate-risk prostate cancer: Results of two prospective trials. Acta Oncol 2013;52:463–9.
- Valery R, Mendenhall NP, Nichols RC, Jr., Henderson R, Morris CG, Su Z, et al. Hip fractures and pain following proton therapy for management of prostate cancer. Acta Oncol 2013;52:486–91.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030 J Bone Joint Surg Am 2007;89:780–5.

