Angiosarcoma (AS) is a rare and often high-grade malignant vascular tumor [Citation1]. They represent below 2% of all soft tissue sarcomas and present in a broad variety of anatomic locations [Citation1,Citation2]. Predisposing factors include lymphedema (Steward-Treves syndrome) and radiation, and cutaneous post-irradiation AS has become a well known complication to breast conserving therapy [Citation3,Citation4].
Traditional treatment of AS constitutes radical surgical resection as the only potentially curative treatment. Radiotherapy after resection has proven to correlate with better outcome [Citation4,Citation5]. Even so both local and distant recurrence is often seen [Citation5] and overall survival of AS patients is reported to be around 2.5 years with a five-year survival of approximately 30% [Citation1,Citation5]. For metastatic cases, anthracyclins usually in the form of pegylated doxorubicin is considered to be the treatment of choice [Citation6]. In a resent retrospective analysis of AS patients with disease below the clavicle, paclitaxel proved efficient with response rates of 58% [Citation1,Citation4].
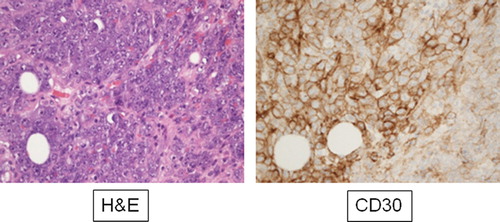
CD30 is a transmembrane glycoprotein in the tumor necrosis factor receptor super-family affecting multiple cytokine pathways. It is expressed on activated T- and B-cells and is a diagnostic marker of some lymphomas [Hodgkin's lymphoma, Anaplastic large cell lymphoma (ALCL), some B-cell lymphomas]. Though not part of standard diagnostic immunohisotchemical analysis for sarcomas, high expression of CD30 has been reported in up to 30% of AS's [Citation2,Citation3,Citation7,Citation8], making this a potential diagnostic pitfall in AS's as well as opening up to potentially new treatment possibilities with targeted therapy using antibody drug conjugates.
Brentuximab is an anti-CD30 antibody drug conjugate that is highly efficient in introducing cell death in CD30 positive cells [Citation9]. It has shown response rates around 60% in patients with Hodgkin's lymphoma and ALCL and is now considered standard of care in relapse or refractory Hodgkins lymphoma and ALCL [Citation10]. It was suggested therefore that brentuximab could be used to treat CD30 positive AS's [Citation2] though this was never tested in a clinical trial.
The Danish medical counsel has established a Second Opinion committee, which can be approached by doctors on behalf of their patients. It grants permission to the use of off-label drugs on experimental basis outside of clinical trials (Danish Medical Counsel Website information: https://sundhedstyrelsen.dk/da/sundhed/behandling-og-rettigheder/eksperimentielbehandling).
Based on the above mentioned biological considerations we have tested brentuximab (1.8 mg/kg every two weeks) in a patient with confirmed CD30 positive AS who showed progression on standard therapy. The detailed clinical course is described below.
Case report
A 59-year-old woman was diagnosed in 2004 with infiltrative ductal carcinoma of the right breast. She underwent breast conserving surgery followed by adjuvant chemotherapy (seven cycles of cyclophosphamide, epirubicin and 5-flouro-uracil) and radiotherapy of the breast (48 Gy at 24 fractions, plus a boost to the tumor-bed with 10 Gy in 5 fractions). This was followed by hormonal therapy in the form of tamoxifen 20 mg/d for five years. Regular follow-up showed no evidence of disease.
In July 2010 she presented with a diffuse, discolored tumorous infiltration in the irradiated breast. Punch biopsy revealed CD30 + AS [Citation3] which was considered radiation-induced. She was treated with mastectomy of the right breast without further adjuvant chemo- or radiotherapy. In the course of the following three years she developed multiple local recurrences that were treated with multiple attempts at radical surgical resection including thoracoplasty. In August 2013 computed tomography (CT) scan revealed yet another local recurrence as well as metastasis to one internal mammary lymph node. The patient was treated with standard salvage chemotherapy. As she previously received epirubicin as part of her adjuvant chemotherapy for carcinoma of the breast, she was treated with weekly paclitaxel. The first F-18 FDG PET/CT in November 2013 showed regression of size and reduced FDG uptake. In March 2014 (following eight cycles) treatment was discontinued due to CT confirmed 40% increase in tumor size.
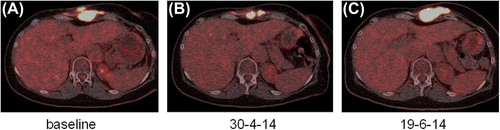
As the tumor was CD30 positive (), permission to experimental treatment with brentuximab (1.8 mg/kg every two weeks) was granted by the national second opinion committee. Treatment response was evaluated by F18 FDG-PET/CT scan and initiated March 2014 (). First control scan May 2014 after two cycles of treatment showed regression of the thoracic tumor size and reduced FDG uptake (). The second control scan however showed progressive disease () and treatment was discontinued after four cycles. Following brentuximab the patient received pazopanib. The first status scan showed further progression and treatment was discontinued.
Discussion
To the best of our knowledge this is the first clinical report describing the use of brentuximab in patients with progressing metastatic CD30 positive AS. Our patient had progressive disease before starting brentuximab. The first scan following two cycles showed reduction in both FDG uptake and tumor size. Given that the patient had more than a 40% increase in tumor size just before the start of brentuximab, a stabilization of disease progression should be considered as a sign of effective therapy. The response was however short lived, as the second scan almost three months after treatment initiation showed progressive disease. The pathologist estimated that 40% of the tumor cells showed CD30 positivity. This may have contributed to the short duration of response, as it is possible that response rates correlate with the extent of CD30 expression in tumor cells, and that a higher expression would lead to better and more sustained response. It is further to be noted, that the patient also showed a relatively short-lived response to paclitaxel, with tumor progression after eight cycles (two months) and did not respond to pazopanib treatment at all. Moreover brentuximab was administered as a single agent treatment, and it is possible that brentuximab may prove more efficient when administered in combination with other chemotherapeutic agents.
This transient response to the drug, though moderate and unsustained, can be considered a proof of principle suggesting a possible efficacy of this drug in similar cases. We recommend this drug to be tested in a formal phase II trial of other CD30 positive sarcomas.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Fury MG, Antonescu CR, Van Zee KJ, Brennan MF, Maki RG. A 14-year retrospective review of angiosarcoma: Clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J 2005; 11:241–7.
- Alimchandani M, Wang ZF, Miettinen M. CD30 expression in malignant vascular tumors and its diagnostic and clinical implications: A study of 146 cases. Appl Immunohistochem Mol Morphol 2014;22:358–62.
- Aggerholm-Pedersen N, Baerentzen S, Holmberg Jorgensen JP, Safwat A. A rare case of CD30(+), radiation-induced cutaneous angiosarcoma misdiagnosed as T-cell lymphoma. J Clin Oncol 2011;29:e362–4.
- Schlemmer M, Reichardt P, Verweij J, Hartmann JT, Judson I, Thyss A, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: A retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer 2008;44: 2433–6.
- Mark RJ, Poen JC, Tran LM, Fu YS, Juillard GF. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer 1996;77:2400–6.
- Young RJ, Natukunda A, Litiere S, Woll PJ, Wardelmann E, van der Graaf WT. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: Pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer 2014;50:3178–86.
- Weed BR, Folpe AL. Cutaneous CD30-positive epithelioid angiosarcoma following breast-conserving therapy and irradiation: A potential diagnostic pitfall. Am J Dermatopathol 2008;30:370–2.
- Lin CF, DeFrias D, Lin X. Epithelioid angiosarcoma: A neoplasm with potential diagnostic challenges. Diagn Cytopathol 2010;38:154–8.
- Kim KM, McDonagh CF, Westendorf L, Brown LL, Sussman D, Feist T, et al. Anti-CD30 diabody-drug conjugates with potent antitumor activity. Mol Cancer Ther 2008;7:2486–97.
- Ansell SM. Brentuximab vedotin. Blood. 2014.