Abstract
Background. A phase Ia/b dose-escalation study was performed to characterize the safety, efficacy and pharmacokinetic properties of the oral small molecule insulin-like growth factor-1-receptor pathway modulator AXL1717 in patients with advanced solid tumors.
Material and methods. This was a prospective, single-armed, open label, dose-finding phase Ia/b study with the aim of single day dosing (phase Ia) to define the starting dose for multi-day dosing (phase Ib), and phase Ib to define and confirm recommended phase II dose (RP2D) and if possible maximum tolerated dose (MTD) for repeated dosing.
Results and Conclusion. Phase Ia enrolled 16 patients and dose escalations up to 2900 mg BID were successfully performed without any dose limiting toxicity (DLT). A total of 39 patients were treated in phase Ib. AXL1717 was well tolerated with neutropenia as the only dose-related, reversible, DLT. RP2D dose was found to be 390 mg BID for four weeks. Some patients, mainly with NSCLC, demonstrated signs of clinical benefit, including four partial tumor responses (one according to RECIST and three according to PET). The 15 patients with NSCLC with treatment duration longer than two weeks with single agent AXL1717 in third or fourth line of therapy showed a median progression-free survival of 31 weeks and overall survival of 60 weeks. Down-regulation of IGF-1R on granulocytes and increases of free serum levels of IGF-1 were seen in patients treated with AXL1717. AXL1717 had an acceptable safety profile and demonstrated promising efficacy in this heavily pretreated patient cohort, especially in patients with NSCLC. RP2D was concluded to be 390 mg BID for four weeks. Trial number is NCT01062620.
The IGF-1 receptor (IGF-1R) signaling pathway is believed to be critical for cancer cell growth, survival, metastasis [Citation1], and resistance to therapy [Citation2], and to protect tumor cells from chemotherapy and irradiation [Citation1]. IGF-1R is prominently expressed in non-small cell lung cancer (NSCLC) [Citation3]. Numerous clinical trials have been completed to explore the anti-tumor potential of anti-IGF-1R antibodies or IGF-1R tyrosine kinase inhibitors, mostly in combination with other agents with anti-tumor activity [Citation4]. To date, phase III studies have however failed to confirm efficacy of these agents [Citation5]
AXL1717 is identical to picropodophyllin (PPP) and was identified as an inhibitor of IGF-1R signaling in a cellular screen [Citation6]. AXL1717 has shown pronounced antitumor activity including tumor regression against a wide range of cancers in relevant animal models [Citation6,Citation7]. In contrast, AXL1717 was ineffective in tumor models whose growth was driven by specific oncogenes, like VSRC and HER2 [Citation6,Citation8]. It has also been reported that AXL1717 sensitizes tumor cells to cytostatic agents [Citation9] and irradiation [Citation10]. Mechanistic in vitro studies have demonstrated that incubation with AXL1717 induces degradation of the IGF-1R, reduces the level of phosphorylated IGF-1R and the activity of proteins downstream in the signaling pathway [Citation6,Citation11], and in particular has no detectable effects on closely related receptors including the insulin receptor [Citation6,Citation12].
Recently it has been realized that AXL1717 has additional effects unrelated to the effect on IGF-1R signaling [Citation13]. For a long time AXL1717 was believed to yield arrest in the G2/M phase of the cell division, but it has now been shown that tumor cells are arrested in mitosis and that this is associated with inhibition of microtubule dynamics [Citation14–16]. AXL1717 does not bind beta-tubulin and the molecular target has not been reported [Citation6]. Based on the absence of direct binding to beta-tubulin and on the benign clinical side effect profile, the effect of AXL1717 on microtubule seems to differ from microtubule inhibitors presently in clinical use [Citation17,Citation18]. Thus, in contrast to previous IGF-1R pathway targeting agents, AXL1717 has a second anti-tumoral effect, which may contribute both to efficacy and side effect profile.
The present study reports the safety and efficacy of the IGF-1R pathway modulator AXL1717 in patients with advanced solid tumors lacking available treatment options, with a special emphasis on patients with NSCLC. The purpose of the phase Ia part of the clinical study was to define the starting dose for phase Ib and of phase Ib to define and confirm RP2D, and if possible, identify MTD for repeated dosing. Secondary objectives included establishing the tolerability and adverse event (AE) profiles, pharmacodynamic effects, and preliminary antitumor activity.
Material and methods
Patients
Patients, age 18 years or older, with histologically confirmed diagnosis of advanced solid or hematological malignancy without remaining treatment options, with a life expectancy of at least three months, were eligible for study entry provided they met the following entry criteria: Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less, adequate hematology, blood chemistry, and renal and liver function. Exclusion criteria were known malignancy in CNS, prior anti-tumor therapy within four weeks from enrolment and/or radiotherapy (given palliatively at non-target lesions) within one week prior to study drug administration day. Based on preclinical data in animal studies where no adverse effects on blood glucose were observed, diabetes mellitus was not an exclusion criterion. All patients provided signed informed consent, and local ethics committee approval was obtained. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
Study design and procedures
This was a prospective single institution, single-armed, open label, dose finding phase Ia/b study. The purpose of phase Ia (single day BID dosing) was to define the starting dose for phase Ib (repeated BID dosing) and of phase Ib (repeated BID dosing) to define in 3–6 patients and confirm the recommended phase 2 dose (RP2D) in another 10–20 patients and if possible maximal tolerated dose (MTD) for repeated dosing (up to 28 days). An oral suspension of PPP (AXL1717) was used in the study (supplied by Axelar AB, Stockholm, Sweden). Patients were treated with increasing doses of AXL1717 with a starting dose in phase Ia of 5 mg BID. The starting dose was defined based on toxicological findings in animal studies. The dose was then escalated in 100% steps up to 80 mg BID and thereafter increased in steps of 50% and then in steps of 33% until the top dose of 2900 mg BID was reached according to a modified Fibonacci schedule [Citation19]. Patients were treated BID during one day (morning and evening) followed by a three-week observation period. If no dose-limiting toxicity (DLT) was observed during the observation period during the first cycle of a patient, then the next patient could be included on the next higher dose level. All patients could be individually dose escalated until first DLT or tumor progression at the discretion of the investigator. All dose escalation decisions were taken by the data safety monitoring board (DSMB) including independent member.
The starting dose in phase Ib was 700 mg BID for seven consecutive days followed by a three-week observation period. The starting dose was based on the absence of DLTs in Phase Ia also at the highest tested dose level of single day 2900 mg BID. Full data was presented to the Regulatory Authorities (RA) and the starting dose was decided in collaboration with RA. The starting dose in phase Ib was based on a safety factor of 4 that was introduced to compensate for the prolongation of treatment. The objective was to identify a suitable dose for 28 days of continuous BID dosing. Increasing doses of AXL1717 were given until two or more out of 3–6 patients at one dose level experienced DLT after their first (pivotal) treatment (≥ 33% of the patients reporting DLTs), thus constituting the MTD. The recommended phase 2 dose was however decided by DSMB after reviewing the occurrence of DLTs following all treatment cycles and accounting for all available safety information. DLT was defined as the occurrence of any CTCAE (Common Terminology Criteria for Adverse Events, version 4.0 used) grade 3 or higher related clinically relevant AE, however, grade 4 was required for neutropenia and leukopenia. When MTD was reached, the next lower dose level was further explored. First, seven-day dosing was explored until MTD was reached, then 14-day, 21-day and 28-day dosing was explored in consecutive order.
The recommended phase 2 dose (RP2D) was defined as a dose that was tolerated well by six patients. Following identification of RP2D, this dose was further explored in an increased patient population (RP2D confirmation phase). This confirmation phase of four weeks treatment with 390 mg AXL1717 BID also allowed treatment for two periods of four weeks after approval of an amendment within the study protocol. A 21-day treatment free observation period followed after last dose in each treatment period with the possibility to prolong the observation period another three weeks until the patient fulfilled the continuation criteria (any related AEs should have resolved to CTCAE grade 1 or better, neutropenia and leukopenia should have resolved to CTCAE grade 2 or better). For some patients enrolled at a later stage, the observation period was reduced to 14 days. Discontinuation criteria included patient preference, AE contraindicating continuation, best interest of patient, tumor progression, failure to fulfil continuation criteria, pregnancy and need for treatment with granulocyte growth factors. Patients without evidence of tumor progression at the end of the study could be treated indefinitely at the discretion of the investigator in a special extension study, in which the dosage was not more intensive than in the main study (dose and treatment duration) and with similar treatment-free periods.
Assessments of biomarkers and efficacy
Biomarker analyses included serum IGF-1, IGF-binding protein 3 (IGFBP-3) and growth hormone (GH). Additionally, blood glucose, insulin, C-peptide, and HbA1c were analyzed to exclude inhibition of the insulin receptor. The density of IGF-1 receptor on the surface of neutrophil granulocytes was assessed with previously published methods [Citation20]. Tumor response was assessed by imaging with computed tomography according to RECIST 1.1 criteria [Citation21] and 18F- Fluoro-Deoxy-Glucose Positron Emission Tomography (FDG PET) [Citation22] as well as by potential biomarkers after two treatment periods in phase Ib and/or at study end and compared to baseline. Radiological response evaluation was not performed in phase Ia since the single day BID dosing was not expected to result in any measurable radiological responses.
Pharmacokinetics
In phase Ia plasma samples were planned to be drawn predose and then 15, 30, 45, 60, 75, 90, 120 (2 h), 180 (3 h), 240 (4 h), 360 (6 h), 480 (8 h) and 720 (12 h) minutes after the morning dose for each treatment cycle. In phase Ib plasma samples were planned to be drawn at the same time points after the morning dose on Day 1, 7, 14, 21 and 28 as appropriate depending on the dosing duration of the cycle. For most profiles the last sample was taken at 10 hours rather than at 12 hours and the actual time points of sampling were used in the analyses. Serum concentration of AX1717 was analyzed by LC-MS/MS as described earlier [Citation23]. Non-compartmental methods were used to describe the concentration-time profiles [Citation24].
Results
Patient characteristics
Sixteen patients were enrolled in phase Ia while 39 patients were enrolled in phase Ib. Of these 39 patients, six were enrolled during phase Ia and continued their treatment in phase Ib. A separate cohort was formed from the patients with NSCLC (19 patients out of whom two patients participated in phase Ia only). The patients enrolled in phase Ia (nine male and seven females) had a median age of 58.5 years (range 25–83 years), and in phase Ib (24 male and 15 females) median age 64 years (range 27–80 years). Nineteen patients with NSCLC, seven patients with prostate cancer, five with colorectal cancer and five with malignant melanoma represented the most common diagnoses. The patients were heavily pretreated prior to entry into the study. Further demographic characteristics of the study cohorts are summarized in .
Table I. Baseline patient demographics and clinical characteristics of the entire cohort.
Study treatment
In phase Ia, the dose was increased both between and within patients from the starting dose of 5 mg BID to the top dose of 2900 mg BID. The patients received 1–14 treatment periods. In phase Ia, 78 single day treatments (156 administrations) were administered with 99% compliance (Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049290). Based on pharmacokinetic data and the notion that repeated dosing is needed for an effective anti-tumor activity, the starting phase Ib dose was defined to be 700 mg BID for seven days. In total, phase Ib included 68 treatment periods with 1–4 weeks of treatment with a total prescribed dosing of 147 weeks and a total estimated compliance of 96.4% (Supplementary Table II, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049290). The compliance in connection with PK sampling at site was 100%. The 13 patients entering the extension study were treated for a total of 111 weeks (Supplementary Table III, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049290).
Safety
Patients that had received at least one dose of study medication were included in the safety analyses. In phase Ia, single day treatment with AXL1717 was well tolerated; the dose was successfully escalated from 5 mg BID to 2900 mg BID without any DLT, any CTCAE grade 3 or higher related AE or any study drug-related withdrawal. During phase Ia, 22 serious adverse events (SAE) were reported out of which six were fatal. All these events complied with the expected scenario for the underlying tumor disease/progression and at no occasion was a SAE assessed related to the study treatment. In phase Ib, the first DLTs were reported and also the first suspected unexpected serious adverse reactions (SUSAR). Twenty-six patients reported any AE that were possibly or probably related to the study drug (). Seventy-eight events have been assessed as related to the study drug (either possibly; 37 events or probably; 41 events). Fifteen non-fatal SAEs were reported by 13 patients out of which seven events were assessed as possibly/probably related to study medication. One patient developed a pustular rash at a dose of 930 mg BID that resolved, and the other six events were related to neutropenia. All events were reversible. Six fatal cases within 30 days of last dose were reported and five of these cases were unlikely related to study drug. One patient had a probably related neutropenia in a fatal event with the underlying disease as the likely cause of the fatal outcome. Neutropenia, the main DLT, was reversible and dose-related and occurred in 26% of the patients in phase Ib.
Table II. Possibly/probably related adverse events in phase Ib.
The first cohort in the Phase Ib part of the study was treated with one-week of 700 mg AXL1717 BID. As no patients reported DLTs in their first cycle of treatment, the next cohort was included on the one-week 930 mg BID level. Two of the four patients (50%) treated on this dose level reported DLTs (febrile neutropenia). Due to the severity of the DLTs in the 930 mg BID cohort and taking into account that the objective was to find a dose for continuous dosing, the next cohort was entered on the one-week 520 mg BID (two dose levels reduction) following a decision of DSMB. Two out of a total of seven patients (29%) reported DLTs on this dose level (neutropenia and combination of neutropenia and thrombocytopenia) which as assessed as acceptable. The next cohort was two-week 520 mg BID. A total of three out of seven patients (43%) on this level reported DLTs (all neutropenias which in one case developed into febrile neutropenia), which was assessed as unacceptable and the next cohort was therefore three-week 390 mg BID. One DLT of febrile neutropenia was reported in the five patients in this cohort. The following patients were treated with four-week 390 mg BID of AXL1717. A total of 12 patients treated with a total of 18 treatment periods were included in the study. No patients reported a DLT on this level. The RP2D was thus identified to minimize the frequency of neutropenia and for continuous 28-day single agent dosing was identified as 390 mg BID. Three related events of thrombocytopenia (four in total) occurred during the study, all of them in patients with simultaneous severe neutropenia. In two cases, neutropenia developed in connection with severe constipation, possibly causing increased absorption due to intestinal delay. This motivates special attention directed to constipation problems and other situations that may increase the intestinal transit time when planning future studies.
Efficacy
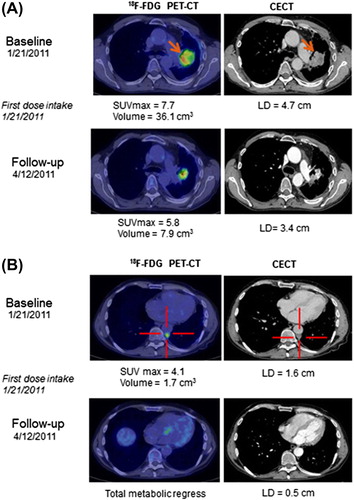
Tumor response according to RECIST was not assessed in the phase Ia part of the study, but it was observed that patients with NSCLC survived longer than expected and some of them showed prolonged duration of stable disease as previously published [Citation26]. Eight patients were withdrawn from the study prior to study assessments, mainly due to tumor progression. Evaluation at the end of the study showed that 15 patients had progressive disease (PD), 10 patients stable disease (SD), and three patients non-measurable disease (all prostate cancer patients). In addition, four patients had partial tumor responses during the study (including the extension study); three with NSCLC [one according to RECIST 1.1 (including PR on FDG PET) and two on FDG PET] and one with prostate cancer (on FDG PET). The median overall survival (OS) for the whole population of 49 patients was 30 weeks, with one patient alive at cut-off (). All patients in phase Ib treated for more than two weeks had a median OS of 42 weeks and progression-free survival (PFS) of 13 weeks (). At cut-off, one of these 34 patients was still alive. Early signs suggested that NSCLC patients might have clinical benefit of treatment with single agent AXL1717 [Citation1]. This cohort of 15 patients with treatment duration of more than two weeks was followed separately; Kaplan-Meier assessment showed a median OS of 60 weeks and a median PFS of 31 weeks (). None of the 15 NSCLC patients were still alive at cut-off. One patient with NSCLC had a remarkable tumor response () with three index lesions at baseline and at the three months assessment measured with FDG PET-CT, the functional tumor volume was reduced with 81% in total and with 100% in two out of the three tumor locations, showing extensive reduction of tumor metabolism. The RECIST assessment with CT scan was overall reduced with 64% at the three-month time point.
Figure 1. Kaplan-Meier plot for the overall survival of the total population (n = 49) with one patient alive at cut-off.
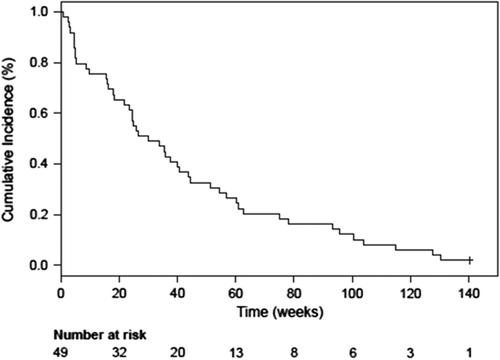
Figure 2. Kaplan-Meier plot for the overall survival (with one patient alive at cut-off) and the progression-free survival of the total population in multidose (Phase Ib) with a treatment duration longer than 2 weeks with single agent AXL1717 (n = 34).
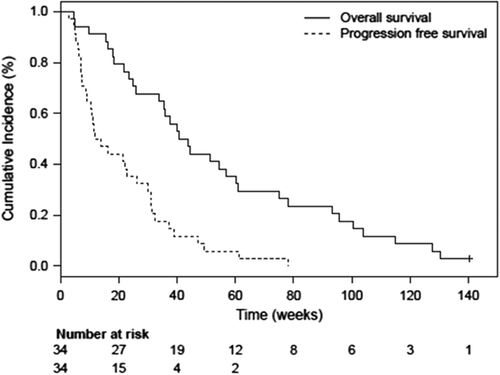
Figure 3. Kaplan-Meier plot for the overall survival (with no patients alive at cut-off) and progression-free survival of the 15 patients with NSCLC with treatment duration longer than 2 weeks with single agent AXL1717.
Figure 4. A and B Transaxial images at baseline and 3-month follow-up with FDG PET-CT and contrast-enhanced CT (CECT) in a patient with a primary NSCLC (solid arrows) in the left lung and pleural metastases (cross marked). A) shows a significant reduction in the primary tumor metabolism measured in maximum standardized uptake value (SUVmax, g/mL) and size on CECT evaluated with the RECIST 1.1 criteria (LD, transaxial longest diameter) at 3-month follow-up. B) shows a concordant significant reduction in the pleural metastases with total metabolic regress on FDG PET-CT after treatment.
Pharmacokinetics
After single doses in phase Ia onset of absorption was rapid with measurable plasma concentrations within 15 minutes. Peak concentrations were observed 1–4 hours after drug intake. Cmax and AUC increased less than proportional to dose over the dose range 5–80 mg but were close to dose proportional after higher doses from 120 mg up to 2900 mg. Dose adjusted AUCinf values are shown in Supplementary Figure 1 (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049290). For doses 120 mg and higher the elimination half-life ranged from 1.6 to 4.5 hours (geometric mean by dose level).
After repeated doses in phase Ib the majority of patients demonstrated a larger than expected increase in AUC from Day 1 to subsequent dosing days at the same dose level. An example is shown in Supplementary Figure 2 (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049290) for a patient receiving 520 mg BID over 14 days. This increase is not explained by accumulation but rather an increase in bioavailability which is fully developed within seven days of dosing. Results for Cmax and AUC0-12 representing the dose interval are shown in Supplementary Table IV (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049290) for the doses 390 mg BID and 520 mg BID with data for at least five patients on each dosing day.
Pharmacodynamics
Analyses of the potential biomarkers IGF-1 and IGFBP-3 in serum showed that 11 of the 17 patients (65%) with 15 days or longer continuous treatment exhibited increased S-IGF-1 levels following treatment with AXL1717. In contrast, only five out of 20 patients (25%) with 14 days or shorter continuous treatment exhibited increased levels of S-IGF-1 (p = 0.0411, Wilcoxon Test). Eleven of the 17 patients (65%) with 15 days or longer continuous treatment showed increased serum IGFBP-3 levels. In contrast, only four out of 20 patients (20%) with 14 days or shorter continuous treatment exhibited increased levels of serum IGFBP-3 (p = 0.0031). The diagnoses of the 11 patients showing increased IGF1 and IGFBP3 were prostate cancer (n = 5), NSCLC (n = 2), ovarian cancer (n = 1), kidney cancer (n = 1), malignant melanoma (n = 1) and B-cell lymphoma (n = 1), respectively. As the S-IGF1 and S-IGFBP3 concentrations varied largely among the investigated patients (25–332 ng/mL and 1010–5710 ng/mL, respectively), we present the mean differences in IGF1 (Supplementary Figure 3A, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049290) and IGFBP3 (Supplementary Figure 3B, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049290) levels after continuous treatment with AXL1717 for ≤ 14 days and > 14 days by comparing the values with the predose values (at Day 0) for each patients. Using Student's t-test the mean increase in both S-IGF1 and S-IGFBP3 after > 14 days treatment, as compared to shorter treatments (≤ 14 days), was proven statistically significant (p = 0.03 and p = 0.0008, respectively). No significant changes were noted in the analyses of serum GH, insulin, C-peptide, blood glucose or HBA1c levels throughout the study. None of the included patients were diabetic. IGF-1R expression on granulocyte surface was assessed as a potential biomarker. A total of 47 samples with relevant controls were available. Fifteen data sets were from patients treated with AXL1717 for seven days and showed a mean decrease of 23% in IGF-1R expression; 14 data sets from patients treated for 14 days had a mean decrease of 16%; 10 sets from patients treated for 21 days a decrease of 33%; and the mean receptor down-regulation of eight data sets from patients treated for 28 days was 41%. On average, the IGF-1R down-regulation in all 47 samples was 27% (p < 0.01).
Discussion
This is the first clinical study of the small molecule IGF-1R pathway modulator AXL1717. AXL1717 was well tolerated with few study drug-related AEs with treatment at RP2D. Neutropenia was the only dose limiting AE identified in the clinical study, in agreement with findings in previous toxicity studies in dogs and mice. Neutropenia was readily reversible and dose-related.
Even though this phase I study was not designed for assessment of tumor response, some patients, mainly with NSCLC, showed signs of treatment benefit with AXL1717. All NSCLC patients received AXL1717 as single agent therapy in third or fourth line of treatment and all received AXL1717 as intermittent treatment with a non-optimized dose. Among the 15 NSCLC patients with treatment duration longer than two weeks, the median OS was 60 weeks and the median PFS was 31 weeks. Previous phase III NSCLC studies in second-line setting have shown a median OS of 18–20 weeks and a median PFS of 7–10 weeks for best supportive care/placebo [Citation25,Citation26]. However, due to the limited number of patients in the present study, care should however be taken in interpreting these encouraging data. In addition, four patients had partial tumor responses during the study (one according to RECIST and three according to PET); three with NSCLC and one with prostate cancer. NSCLC tumors are known to have a high density of IGF-1R and NSCLC patients were indeed the first to respond to AXL1717 [Citation3,Citation27]. To the best of the authors’ knowledge, no single agent tumor response in NSCLC has been reported previously for any pharmaceutical targeting the IGF-1R pathway [Citation3,Citation28].
Treatment with AXL1717 resulted in clinical effects on the IGF-1R pathway. First, granulocytes showed reduced amounts of surface exposed IGF-1R, suggesting induced degradation of IGF-1R similar to in vitro observations [Citation31]. Furthermore, there was a clear trend for enhanced serum IGF-1 in patients treated with AXL1717 for 15 days or longer continuous treatment, although modest. Serum IGF-1 and IGFBP-3, biomarkers affected by IGF-1 receptor inhibition [Citation4], have been shown to increase in patients with multiple myeloma and solid tumors following treatment with the anti-IGF-1 receptor antibody figitumumab [Citation16,Citation20]. In agreement with AXL1717, treatment with figitumumab yields down-regulation of IGF-1R, suggesting that this is a relevant comparator [Citation30]. Importantly, there is no evidence that increase in the serum IGF-I induced by treatment with the anti-IGF-1R antibody figitumumab was sufficient to overcome the desired IGF-1R inhibition [Citation31]. This was suggested to be due to a parallel elevation of the levels of IGFBPs that reduce IGF bioactivity [Citation31]. However, the modest increases in serum IGF-1 and IGFBP-3 seen in the majority of patients treated with AXL1717 for more than two weeks contrast with the major changes in serum IGF-1 following treatment with figitumumab [34]. AXL1717 clearly interacts in a different way with the IGF-1R signaling pathway compared with that of the anti-IGF-1R antibodies, which directly block binding of IGF.
No treatment related effects were seen with respect to parameters connected with insulin receptor inhibition such as serum glucose, insulin, C-peptide or B-HbA1c. Blood glucose changes appear to be an IGF-1R inhibitor class effect for the anti-IGF-1R antibodies, and mild increases in blood glucose levels have been reported in approximately 25% of treated patients [Citation4]. These antibodies, in analogy with AXL1717, do not cross-react with the insulin receptor and it has been speculated that an increased gluconeogenesis and insulin resistance caused by growth hormone secondary to loss of negative feedback at the pituitary by IGF-1R inhibition may be responsible for the appearance of hyperglycemia [Citation32]. Notably, most patients treated with IGF-1R inhibitors in single-agent studies did not develop severe hyperglycemia, probably due to a compensatory increased insulin secretion. Thus, other factors such as background diabetes and particularly the use of low-dose corticosteroids as premedication for chemotherapy are likely contributors to the incidence of hyperglycemia in IGF-1R inhibitor/chemotherapy combination studies. In the present study, the heterogeneity of this severely advanced cancer population makes interpretation challenging.
Most monoclonal antibodies directed against IGF-1R bind to the extracellular domain of the receptor to block ligand binding and receptor activation, whereas previously described small anti-IGF-1R molecules are tyrosine kinase inhibitors binding to the intracellular catalytic domain. AXL1717 works by a different mechanism and affects not only the IGF-1R but also interferes with microtubule dynamics [Citation14–16]. This second effect of AXL1717 may explain the occurrence of neutropenia seen in this clinical study and suggests that neutrophil counts should be monitored in future clinical trials with AXL1717 as a possible marker of toxicity.
In summary, the present study has shown treatment with single agent AXL1717 to be tolerable in a population of progressive, advanced and refractory cancer patients without remaining treatment options. Reversible dose-related neutropenia was found to be the main DLT. The recommended phase 2 dose for continuous 28-day single agent dosing was identified as 390 mg BID. Clinical benefit was observed in patients with NSCLC despite the phase I safety design of the present study. Prospective randomized trials have been initiated to further evaluate the promising results of the present study.
Supplementary material available online
Supplementary Figures 1–3 and Tables I–IV available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049290
ionc_a_1049290_sm0929.pdf
Download PDF (416.3 KB)Acknowledgements
This work was supported by Axelar AB, Stockholm, Sweden.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Corvaia N, Beck A, Caussanel V, Goetsch L. Insulin-like growth factor receptor type I as a target for cancer therapy. Front Biosci (Schol Ed) 2013;5:439–50.
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 2008;8:915–28.
- Karp DD, Paz-Ares LG, Novello S, Haluska P, Garland L, Cardenal F, et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol 2009;27:2516–22.
- Scagliotti GV, Novello S. The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors. Cancer Treat Rev 2012;38:292–302.
- Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: An update. Nat Rev Cancer 2012;12: 159–69.
- Girnita A, Girnita L, del Prete F, Bartolazzi A, Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res 2004;64:236–42.
- Vasilcanu R, Vasilcanu D, Rosengren L, Natalishvili N, Sehat B, Yin S, et al. Picropodophyllin induces downregulation of the insulin-like growth factor 1 receptor: Potential mechanistic involvement of Mdm2 and beta-arrestin1. Oncogene 2008;27:1629–38.
- Liu BY, Soloviev I, Huang X, Chang P, Ernst JA, Polakis P, et al. mammary tumor regression elicited by wnt signaling inhibitor requires IGFBP5. Cancer Res 2012;72:1568–78.
- Duan Z, Choy E, Harmon D, Yang C, Ryu K, Schwab J, et al. Insulin-like growth factor-I receptor tyrosine kinase inhibitor cyclolignan picropodophyllin inhibits proliferation and induces apoptosis in multidrug resistant osteosarcoma cell lines. Mol Cancer Ther 2009;8:2122–30.
- Osuka S, Sampetrean O, Shimizu T, Saga I, Onishi N, Sugihara E, et al. IGF1 receptor signaling regulates adaptive radioprotection in glioma stem cells. Stem Cells 2013;31: 627–40.
- Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 2010;18:683–95.
- Fulzele K, DiGirolamo DJ, Liu Z, Xu J, Messina JL, Clemens TL. Disruption of the insulin-like growth factor type 1 receptor in osteoblasts enhances insulin signaling and action. J Biol Chem 2007;282:25649–58.
- Lin FA, Larsson A, Strömberg T. Multiple antitumor effects of picropodophyllin in colon carcinoma cell lines: Clinical implications. Int J Oncol 2012;40:1251–8.
- Wu X, Sooman L, Wickström M, Fryknäs M, Dyrager C, Lennartsson J, et al. Alternative cytotoxic effects of the postulated IGF-IR inhibitor picropodophyllin in vitro. Mol Cancer Ther 2013;12:1526–36.
- Green CJ, Day M. Insulin-like growth factor 1 acts as an autocrine factor to improve early embryogenesis in vitro. Int J Dev Biol 2013;57:837–44.
- Waraky A, Akopyan K, Parrow V, Strömberg T, Axelson M, Abrahmsén L, et al. Picropodophyllin causes mitotic arrest and catastrophe by depolymerizing microtubules via insulin-like growth factor-1 receptor-independent mechanism. Oncotarget 2014;5:8379–92.
- Ekman S, Frödin J-E, Harmenberg J, Bergman A, Hedlund Å, Dahg P, et al. Clinical phase I study with an insulin-like growth factor-1 receptor inhibitor: Experiences in patients with squamous non-small cell lung carcinoma. Acta Oncol 2011;50:441–7.
- Gascoigne KE, Taylor SS. How do anti-mitotic drugs kill cancer cells? J Cell Sci 2009;122:2579–85.
- Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 2009; 101:708–20.
- Gualberto A, Alsina M, Lacy M, Poutney S, Birgin A, Littman B, et al. Inhibition of the insulin like growth factor 1 receptor by a specific monoclonal antibody in multiple myeloma. J Clin Oncol 2005;23:203s. abstr 3048.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47.
- Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009;50 (Suppl 1):122S–50S.
- Rönquist-Nii Y, Eksborg S, Axelson M, Harmenberg J, Beck O. Determination of picropodophyllin and its isomer podophyllotoxin in human serum samples with electrospray ionization of hexylamine adducts by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879:326–34.
- Rowland M. Clinical pharmacokinetics: Concepts and applications, 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott William & Wilkins; 2011.
- Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095–103.
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123–32.
- Gualberto A, Karp DD. Development of the monoclonal antibody figitumumab, targeting the insulin-like growth factor-1 receptor, for the treatment of patients with non-small-cell lung cancer. Clin Lung Cancer 2009;10:273–80.
- Gualberto A, Pollak M. Clinical development of inhibitors of the insulin-like growth factor receptor in oncology. Curr Drug Targets 2009;10:923–36.
- Vasilcanu R, Vasilcanu D, Rosengren L, Natalishvili N, Sehat B, Yin S, et al. Picropodophyllin induces downregulation of the insulin-like growth factor 1 receptor: Potential mechanistic involvement of Mdm2 and [beta]-arrestin1. Oncogene 2008;27:1629–38.
- Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti–type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res 2005;11:2063–73.
- Lacy MQ, Alsina M, Fonseca R, Paccagnella ML, Melvin CL, Yin D, et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 Receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J Clin Oncol 2008;26:3196–203.
- Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene 2009;28: 3009–21.

