Abstract
Background. Carcinomas and their metastases often retain the keratin patterns of their epithelial origin, and are therefore useful as lineage-specific markers in diagnostic pathology. Recently, it has become clear that intermediate filaments composed by keratins play a role in modulation of cell proliferation, migration, and possibly cancer invasion, factors impacting prognosis in early stage non-small cell lung cancer (NSCLC).
Material and methods. Tumor tissue from a retrospective Danish cohort of 177 patients with completely resected NSCLC, stage I-IIIA tumors, were analyzed for keratin 7 (K7) and keratin 34βE12 expression by immunohistochemistry and validated in a comparable independent Norwegian cohort of 276 stage I-IIIA NSCLC patients.
Results. Based on keratin 34βE12/K7 expression, three subgroups with significantly different median cancer-specific survival rates were identified (34βE12+/K7+, 168 months vs. 34βE12+/K7+, 73 months vs. 34βE12-/K7+, 30 months; p = 0.0004). In multivariate analysis, stage II-IIIA (HR 2.9), 34βE12+/K7+ (HR 1.90) and 34βE12-/K7+ (HR 3.7), were prognostic factors of poor cancer-specific survival (CSS) (p < 0.001). Validation in the Norwegian cohort confirmed that stage II-IIIA (HR 2.3), 34βE12+/K7+ (HR 1.6), and 34βE12-/K7+ (HR 2.0) were prognostic factors of poor CSS (p < 0.05). Multivariate Cox proportional-hazard analysis demonstrated that 34βE12+/K7 + and 34βE12+/K7 + status was significantly associated with poor overall survival (p < 0.05).
Conclusion. Keratin 34βE12/K7 expression is a prognostic parameter in resected early stage NSCLC that allows identification of high-risk NSCLC patients with poor cancer-specific and overall survival.
Lung cancer is the leading cause of cancer-related deaths in the world, with a five-year survival rate of 8–15% [Citation1]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases. The main histological subtypes are of epithelial origin and classified as squamous cell carcinoma, adenocarcinoma, or large cell carcinoma. The poor survival rate is primarily due to the usually late stage at diagnosis and a high frequency of recurrence [Citation1]. Around 20–25% of patients diagnosed with early stage NSCLC are suitable for potentially curative resection, but despite progress in multimodal treatments, the risk of recurrence is still pronounced even in early-stage settings [Citation2]. The potential benefit of adjuvant treatment is 5–15% depending on disease stage. Today, patients with stage II-IIIA disease are offered adjuvant cisplatin-based chemotherapy, while adjuvant therapies remain controversial in stage I disease despite the fact that 30–40% of stage I patients experience recurrences [Citation3]. These circumstances emphasize the need for not only predictors of chemotherapy efficacy but also prognosticators to distinguish between patients with high and low risk of recurrence.
Epithelial cancers are characterized by the expression of specific keratin proteins associated with the respective cell type of origin. Keratins play a major role in the cytoskeletal construction, and virtually all epithelial tissues, including the respiratory tract, contain keratins, formerly referred to as cytokeratins. They are the largest group of intermediate filament (IF) proteins, and contain type I (acidic, CK9-CK23) and type II (neutral/basic, CK1-CK8) subclasses, which form heterodimeric pairing between type I and type II subtypes in a preferential manner [Citation4]. The expression of keratin proteins is highly regulated during fetal development and cell differentiation. Keratin 5 (K5) and 14 (K14) are expressed in the basal layers of stratified squamous cell epithelia, and basal cells in various glands and mucosal linings, including the bronchial epithelium. In normal cell formation, K5 and K14 expression is lost during the differentiation process, and the luminal cells express keratin 7 (K7) instead [Citation5]. Carcinomas and their metastases often retain the keratin patterns of their epithelial origin, and have therefore been used routinely as lineage-specific markers for diagnostic pathology.
In lung cancer, expression of K7, keratin 8 (K8), keratin 18 (K18), and keratin 19 (K19) have been reported in adenocarcinomas, while keratin types related to the monoclonal antibody 34βE12, keratin 1 (K1), keratin 5 (K5), keratin 10 (K10), and keratin 14 (K14) are more frequently expressed in squamous cell carcinomas, although conjoined expression may also be present [Citation6]. Expression of keratin 7 is seen in many simple and pseudostratified epithelia, including bronchogenic epithelium. The monoclonal antibody 34βE12 (directed to K1/K10 & K5/K14 and possible other keratins) is useful for detection of high molecular weight keratins and is a sensitive marker of basal and para-basal cells and their proliferation [Citation7]. It primarily recognizes tumors morphologically consistent with squamous cell neoplasm of the lung, although it is also expressed in some tumors morphologically consistent with adenocarcinomas and is almost absent in most small cell lung carcinomas and large-cell neuroendocrine tumors. In addition to their role as diagnostic markers in different cancers, specific keratin expression patterns have recently been associated with functional roles in tumor development, invasion, and metastasis, and also reported to be prognostic indicators in epithelial malignancies [Citation6,Citation8–10].
Considering the importance of keratins in lung cancer, we examined the prognostic effect of keratin expression patterns with regard to cancer-specific survival (CSS) and overall survival (OS) in two independent cohorts of 461 patients. The primary cohort was a retrospective Danish cohort of 177 early-stage NSCLC surgical patients who received no adjuvant or neoadjuvant chemo- or radiotherapy. The results from the Danish cohort were validated in a comparable independent Norwegian cohort of 276 early stage NSCLC surgical patients [Citation11].
Material and methods
Patients, tumors and tissue microarray samples
All patients from the Danish and Norwegian cohorts were early-stage IA-IIIA NSCLC who had undergone radical resection without further neoadjuvant or adjuvant chemotherapy. The histological subtypes had primarily been established in whole section surgical specimens by light microscopy according to World Health Organization criteria and reclassified by a lung pathologist according to the updated pathological classification suggestions [Citation12]. Each case was re-reviewed by a lung pathologist/oncologist according to the revised seventh edition of UICC TNM classification [Citation1].
The initial cohort (Danish)
Tissues, clinicopathological variables and survival data for a consecutive series of NSCLC surgical patients at the University Hospital in Odense, Denmark from 1992 to 1999 were analyzed. Tumor morphology was substantiated according to TTF1, napsin A, p40 and p63 status, and morphology. Synaptophysin and CD56 were evaluated to exclude neuroendocrine etiology. Formalin-fixed and paraffin-embedded (FFPE) tumor blocks were collected, sectioned and stained with hematoxylin and eosin (H&E) to assess tumor content and quality. Four tumor tissue cores of 1 mm were transferred to tissue microarrays (TMAs) using the ATA27 automated arrayer from Beecher Instruments. The TMAs were cut into 4-μm sections using a serial microtome. See Appendix (available online at http://www.informahealthcare.com) for detailed description of the staining process using BenchMark Ultra (Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049291). The detailed methodology has been reported previously [Citation13].
The validation cohort (Norwegian)
The comparable Norwegian validation cohort consisted of 335 early-stage (I-IIIA) NSCLC diagnosed at the University Hospital of North Norway and Nordland Central Hospital from 1990 through 2004 [Citation11]. Tumor morphology was substantiated according to TTF1, p63, and K7. FFPE tumor blocks were collected and evaluated and the most representative areas were transferred using a 0.6 mm diameter tissue-array instrument from Beecher Instruments, Silver Springs MD. The TMA were cut into 4-μm sections using Micron microtome (HM355S); a detailed description has been reported previously [Citation14]. TMA evaluation was validated in 276 of the patients, 47 patients were either not included in the TMA evaluation blocks or the keratin expression of both K7 and CK34βE12 were not available, one patient had no survival data and 11 patients were excluded due to adenocarcinoma in situ reclassification according to the new pathological classification of lung adenocarcinomas [Citation12].
The Danish study was approved by the Ethics Committee of Region Southern Denmark (protocol ID: 20080018) and the Danish Data Protection Agency. The Norwegian cohort was approved by the Regional Committee for Medical and Health Research Ethics (Tromso and Bodo: protocol ID: 2011/2503).
Evaluation of immunohistochemistry
Immunohistochemical stains were examined anonymously using semiquantitative evaluation in light microscopy by a lung pathology specialist. To exclude neuroendocrine morphology, especially in the 34βE12-negative tumors, whole sections of all cases were morphologically evaluated. Keratins 34βE12 and K7 stain were evaluated in the cytoplasm. The number of positive tumor cells was compared to the total number of tumor cells (). Cores were reported as uninformative if the tissue core was lost from the slide or if only a few tumor cells were present (arbitrary cut-off was < 30 tumor cells). If one core was uninformative, then the overall score applied to the remaining cores. For K7, cores with tumor cell content of ≤ 10% staining were considered negative [Citation15]. To exclude basal cell features, tumors with ≤ 1% staining of the tumor cells for 34βE12 were considered negative. The reporting of clinicopathological variables, survival data and biomarker expression were conducted in accordance with the REMARK guidelines [Citation16].
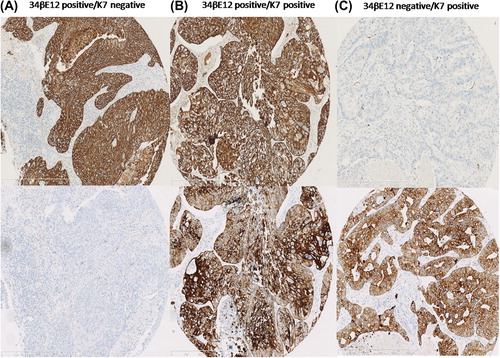
Study end points
The primary endpoint was CSS, defined as the time from date of surgery to date of lung cancer-specific death. Secondary endpoint was OS, from the date of surgery to death of all causes. Patterns of failure after surgery, defined as local and/or distant recurrence or the development of a second primary lung cancer, were registered with date of histological or cytological confirmation or the date of radiological proof when no tumor tissue assessment was available. The follow-up time ended 1 January 2010 in the initial cohort (Danish) and 1 January 2011 in the validation cohort (Norwegian).
Statistical analysis
To examine the associations between molecular marker expression and clinicopathological parameters, the Spearman's rank correlation test and the χ2-test were used. Survival analyses were performed using the Kaplan-Meier method with log-rank (Mantel-Haenszel) tests, and the multiple analyses were performed using Cox proportional-hazard models. Inclusion of variables for the Cox proportional hazard regression model was based on clinical judgment and significance levels of < 0.10 in the exploratory univariate model. Model assumptions were tested using standard methods, and interactions between covariates were assessed. The Cox regression analysis was assessed with estimation of Schoenfeld residuals and Nelson-Aalen estimator for control of proportionality.
Results
Patient characteristics
Demographics with clinical and histopatologic variables from the Danish and Norwegian cohorts are listed in . The initial cohort (Danish) consisted of 177 early-stage NSCLC with pathologically-verified radical surgery. As of January 2010, with a median Kaplan-Meier estimated follow-up time of 165.8 months (3.2–210.8), 92 (52%) of the patients had experienced recurrence and 146 (82%) had died. Of these, 62% died of lung cancer, 10% due to other cancer events, 27% due to non-cancer events, and for 1% the cause of death was not available. Of the 92 patients experiencing recurrence, 69 (75%) were registered with recurrence within the first three years. No patient was lost to follow-up, and the first censoring event in OS was registered after 120 months of observation. The validation cohort (Norwegian) consisted of 276 patients, stage I-IIIA NSCLC. The median Kaplan-Meier estimated follow-up time was 161.7 months (range 0.3–234.0), with 125 (45%) patients experiencing recurrence and 222 (80%) having died.
Table I. Patient demographics and prognostic covariates and cancer-specific survival by univariate log-rank test.
Correlations between keratin expression and clinicopathological factors
Histology and gender were correlated in both cohorts (n = 453). Female gender was associated with adenocarcinoma histology (p < 0.0001, ρ = 0.26). Histology and keratin expression were correlated. Positive 34βE12 expression was associated with squamous cell histology (p < 0.0001, ρ = 0.27) and positive K7 expression was associated with adenocarcinoma histology (p < 0.0001, ρ = 0.58). Expression of 34βE12 were inversely correlated with K7 expression (p < 0.0001, ρ = 0.32). The expression of Keratin 34βE12 and K7 were not correlated with age, differentiation, TNM stage, tumor status (T-stage), or lymph node involvement (N-stage).
Keratin expression and survival analysis
In the Danish cohort, the median OS from surgery to death was 51.2 months, with a 1-, 5 and 10-year survival rate of 92% (95% CI 86–95), 47% (95% CI 39–54), and 31% (95% CI 24–38), respectively. In the Norwegian cohort, the median OS from surgery to death was 47.5 months, with a 1-, 5- and 10-year survival of 81% (95% CI 76–84), 45% (95% CI 40–51), and 28% (95% CI 22–33), respectively.
Analysis of initial cohort
Univariate analyses of covariates associated with cancer-specific death identified stage II-IIIA, adenocarcinoma histology, negative 34βE12 expression, and positive K7 expression as significantly associated with poor CSS (). A multivariate Cox proportional-hazard analysis, including all covariates, demonstrated that stage II-IIIA, negative 34βE12 expression, and positive K7 expression were associated with high risk of CSS (p < 0.05). Moreover, statistical control of possible interactions of covariates revealed a significant interaction between lymph node status and 34βE12 expression (LR test, p = 0.001) and a tendency of interaction with K7 expression (LR test, p = 0.07) (Supplementary Figure 1A-1D, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049291).
We subsequently used pathological TNM stage and divided the patients into subgroups: 34βE12+/K7+, 34βE12+/K7+, and 34βE12+/K7+. The distribution of tumors according to keratin status was 71 (40%) expressing 34βE12+/K7+, 87 (49%) expressing 34βE12+/K7+, and 19 (11%) expressing 34βE12+/K7+. The Kaplan-Meier estimated median CSS for the three subgroups differed significantly: 34βE12+/K7 + median CSS of 2.5 years (95% CI 0.9–4.3), 34βE12+/K7 + median CSS 6.1 years (95% CI 4.1–13.3) and 34βE12+/K7- median CSS of 14 years, (log-rank, p = 0.0003; ). In multiple Cox regression analysis, stage II-IIIA (HR 2.89; 95% CI 1.87–4.46; p < 0.0001), 34βE12+/K7 + status (HR 1.90; CI 1.18–3.05; p = 0.008) and 34βE12+/K7 + status (HR 3.71; CI 1.33–3.42; p < 0.0001) was significantly associated with poor CSS (). The interaction between keratin expression and lymph node status demonstrated that the effect of keratin expression was more profound in lymph node-positive (log-rank, p < 0.0001) than lymph node-negative tumors (log-rank, p = 0.02) ().
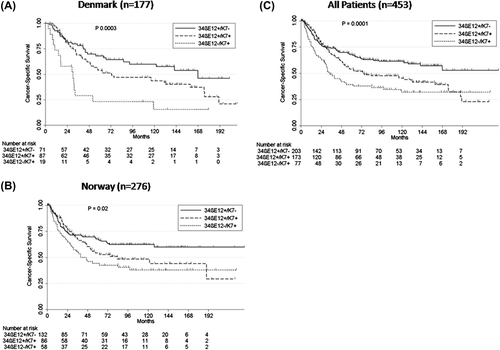
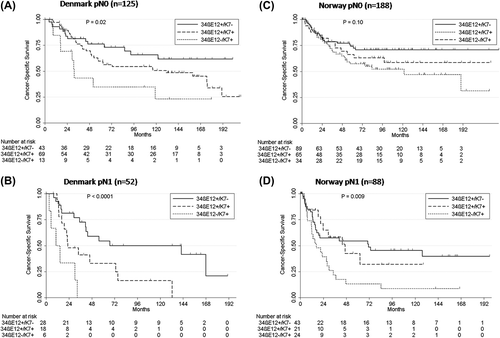
Table II. Results of multiple cox regression analysis and association with significant prognostic factors and cancer-specific survival.
Analysis of validation cohort
Univariate analysis of covariates associated with cancer-specific death in the validation cohort (Norwegian) is listed in . The distribution of tumors according to keratin status was 121 (43%) expressing 34βE12+/K7+, 86 (32%) expressing 34βE12+/K7+, and 58 (22%) expressing 34βE12+/K7+. In 11 (4%) tumors, neither 34βE12 nor K7 were expressed, and the sample size was too small for meaningful comparison. Most of those tumors were squamous cell or large cell carcinomas (LCC) (n = 9; 82%) with CSS comparable to the tumors expressing 34βE12+/K7- ( and Supplementary Figure 2, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049291). The Kaplan-Meier estimated median CSS for the three other subgroups differed significantly: 34βE12+/K7 + median CSS of 3.3 years, 34βE12+/K7 + median CSS 7.0 years (95% CI 4.1–13.3) and 34βE12+/K7+ median CSS was not reached, (log-rank, p = 0.02; ). Multivariate Cox proportional-hazard analysis demonstrated that stage II-IIIA, (HR 2.33; 95% CI 1.56–3.49; p < 0.0001), ECOG performance status 1–2 (HR 1.60; CI 1.09–2.35; p = 0.02), 34βE12+/K7 + status (HR 1.62; CI 1.05–2.50; p = 0.03), and 34βE12-/K7 + status (HR 2.04; CI1.28–3.26; p = 0.003) was significantly associated with poor CSS (). In the Norwegian cohort, a significant interaction between lymph node status and keratin expression (log-rank test, p = 0.0006) was also demonstrated, indicating that the effect of keratin expression was significantly different in tumors without lymph node involvement N0 (log-rank, p = 0.10; ) compared to tumors with N1 or N2 lymph node involvement (log-rank, p = 0.009; ). This was also evident in the combined Danish and Norwegian cohort (log-rank, p = 0.02 N0; , log-rank, p < 0.0001; N1/N2 ). Due to high correlation of histology and keratin expression (p < 0.0001, ρ = 0.71) in the validation cohort, histology was primarily excluded in the validation cohort and examined separately (see below).
Overall survival
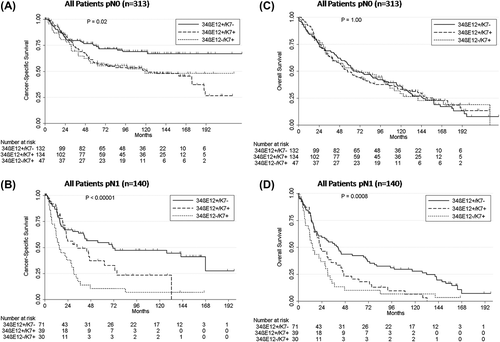
The interaction between keratin expression and lymph node involvement is also evident with respect to OS. In the combined cohorts (n = 453), there were no keratin expression-related survival differences in tumors without lymph node involvement N0 (log-rank, p = 1.00; ), but distinct associations in tumors with lymph node involvement (N1-2) (log-rank, p = 0.0008; ). Multivariate Cox proportional-hazard analysis demonstrated that stage II-IIIA, (HR 1.72; 95% CI 1.39–2.13; p < 0.0001), 34βE12+/K7 + status (HR 1.31; CI 1.04–1.66; p = 0.02), and 34βE12 + /K7 + status (HR 1.38; CI1.04–1.85; p = 0.02) was significantly associated with poor OS ().
Keratin status and histological subtype
Among all the patients (n = 453), 177 (39%) were morphologically adenocarcinomas, 253 (56%) were squamous cell carcinomas and 23 (5%) were large cell carcinomas. Patient demographics and prognostic covariates for adenocarcinomas and squamous cell carcinomas are listed in Supplementary Table II (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049291). In patients with adenocarcinomas, 61 (34%) expressed 34βE12+/K7+, while 109 (62%) expressed 34βE12+/K7 + and seven (4%) tumors expressed, 34βE12+/K7+, respectively. The median CSS for adenocarcinomas with 34βE12+/K7 + was 2.8 years compared to 34βE12+/K7 + 5.2 years, and in tumors expressing 34βE12+/K7+, the median CSS was not reached (log-rank, p = 0.03; Supplementary Figure 3A, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049291).
In tumors morphologically classified as squamous cell carcinomas, 10 (4%) expressed 34βE12+/K7+, while 53 (21%) expressed 34βE12+/K7 + and 190 (75%) expressed 34βE12+/K7+, respectively. The median CSS for squamous cell carcinomas with 34βE12+/K7 + was 1.8 years compared to 34βE12+/K7 + 15.9 years, and in tumors expressing 34βE12+/K7+, the median CSS was not reached (log-rank, p = 0.005; Supplementary Figure 3B available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049291).
Discussion
Keratin IFs are characterized as structural components in epithelial cells. Recently, keratins have been recognized as prognostic indicators in a variety of epithelial cancers [Citation4,Citation6,Citation17]. Our study suggests that specific keratin 34βE12/K7 patterns in lung carcinomas are prognostic factors for CSS and OS in early stage NSCLC. Examination of 453 radically resected, chemotherapy-naïve, lung cancer patients identified three subgroups, 34βE12+/K7+, 34βE12+/K7 + and 34βE12+/K7+, that differed significantly with respect to CSS and OS. In tumors expressing 34βE12+/K7+, the median CSS was 2.9 years, while tumors expressing both keratins 34βE12+/K7 + had a median CSS of 6.3 years, and tumors expressing 34βE12+/K7+ failed to reach the median CSS.
Differences in keratin expression and association with survival was described in a study of squamous cell carcinomas of the esophagus wherein patients with positive expression of K7 and negative K14 expression were associated with a worse OS rate in stage I-IIB; positive K7 expression was particularly associated with a high risk of death, with a hazard ratio of 3.8 (Cox analysis; p = 0.03) [Citation18]. Other studies also reported expression of K7 and 34βE12 relative to patient survival. Proteomic analyses of keratin expression patterns in lung cancer focusing on K7, K8, K18, and K19 in 75 adenocarcinomas identified multiple keratin isoforms that were significantly increased in lung adenocarcinomas compared to normal lung tissue [Citation19]. Positive expression of 34βE12 was also found to be an independent predictor of OS in a study of triple-negative breast cancer patients wherein 34βE12 expression was associated with a good prognosis (p = 0.02) [Citation20]. These findings in different epithelial cancers are in agreement with our study and suggest that patterns of keratin expression may not only reflect the cellular phenotype, but also suggest that the expression patterns are related to cancer prognosis.
Keratin expression patterns were highly correlated with histology; 12% of tumors in the 34βE12+/K7 + subgroup were squamous cell carcinomas compared to 30% in the 34βE12+/K7 + and 93% in the 34βE12+/K7+ subgroup. Although correlated with histology, the keratin expression separates adenocarcinomas into tumors with good and poor prognosis as well as squamous cell carcinomas (Supplementary Figure 3A-B available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049291). Adenocarcinomas with positive expression of both keratins were related to a better prognosis than adenocarcinomas only expressing K7, while squamous cell carcinomas with negative expression of the basal cell keratin 34βE12 were related to a poor prognosis. This indicates that epithelial lung tumors with basal cell expression have a less aggressive course than tumor cells with more luminal K7 expression. A possible explanation could be that loss or alteration of basal cell adhesion and architecture into a more luminal phenotype may play a role in tumor progression. This phenomenon was previously described in the early progression of prostate cancer [Citation21]. Loss of keratin expression and downregulation of cell-cell and cell-matrix contacts play a major role in the epithelial-mesenchymal transition (EMT), which is believed to increase migration and invasion of metastatic tumor cells. A recent study demonstrated a higher invasiveness and growth of keratin-free cells in 3D assay, while re-expression of a small amount of the keratin pair K5/K14 reverses the invasion [Citation9]. This suggests that basal cell keratins assist in the maintenance of an epithelial phenotype that is less likely to acquire metastatic potential.
No additional oncological treatment was administered to any of the patient cohorts, which eliminated potential differences in survival outcome due to different treatment regimes. The long follow-up period with limited censoring, no loss of patients during follow-up, and use of an independent Norwegian validation cohort also strengthen this study. The retrospective design is, however, a limitation. K7 and 34βE12 are standardized antibodies routinely used in many diagnostic assessments and the stainings are therefore easily applied in daily routines. The use of dichotomous cut-off levels for both K7 and 34βE12 is debatable, and antibody staining could be impacted by differences in formalin fixation, concentration, pH, buffers and duration of exposure. One could speculate that the clear prognostic effect of the identified keratin expression profiles may not be of relevance in a current early stage NSCLC population, due to the fact that NSCLC stage (Ib), II and IIIA receive adjuvant chemotherapy. The effect of adjuvant chemotherapy could indeed be dependent on histology and other factors as suggested in the subgroup analysis of the ANITA trial [Citation22]. The subset analysis of the adjuvant phase III study of cisplatin/vinorelbine versus observation (ANITA trial) demonstrated a poor five-year OS rate of adenocarcinomas with a median survival of 37.3 months compared to 45.5 months for non-adenocarcinoma. In the treatment arm however, it was reversed by the administration of adjuvant chemotherapy. The effect of adjuvant chemotherapy cannot be addressed in this study. However, this does not change the prognostic effect of keratin expression albeit an evaluation in adjuvant settings could be highly relevant.
A potential concern of using 34βE12 is a possible irregular distribution in lung cancers other than squamous cell, and in general the use of 34βE12 per se is a pool of high molecular weight keratins (HMWCK). With regard to the first concern, we examined heterogeneous expression of the keratin using the intra class correlation coefficient (ICC) and Spearman adjusted for histology and found ICC ≥ 0.81, and a mean Spearman of 0.91. We then analyzed the ICC and mean Spearman exclusively for adenocarcinomas and found ICC ≥ 0.82 and a mean Spearman of 0.80 [Citation13]. This indicated no evidence of a more heterogeneous 34βE12 staining in non-squamous cell carcinomas. As for the second concern, many antibodies can be open to discussion, including 34βE12, although it is widely used as a marker of basal or para-basal cells and their proliferations in many epithelial cancer studies, including lung cancer [Citation23,Citation24]. The Norwegian validation cohort consisted of 335 patients, earlier described in an article by Donnem et al. [Citation11]. In the present 276 (82%) of these patients were evaluated, while 59 (18%) patients with missing information were left out. In 47 (80%) cases there were no TMA to evaluate, in one case there were no survival data and 11 patients (19%) were excluded due to adenocarcinoma in situ according to the new pathological classification of lung adenocarcinomas [Citation12]. To exclude confounding effects we compared the original Norwegian cohort (n = 335) with the “modified” Norwegian cohort (n = 276) (Supplementary Table I available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049291). There were no significant differences in the distribution of age, gender, histology, EGOG, TNM-stage, lymph node involvement, t-status, vascular infiltration, differentiation, or surgical procedure. There were no significant changes in median CSS and five-year survival. There was however, a minor change in pTNM stage due to reclassification which is also reported in a recently article by Donnem et al. [Citation25]. The changes in TNM did neither influence the median survival nor the CSS (Supplementary Table I available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049291). Consequently, we believe that in most cases (n = 48) the missing information are random, although in 11 cases the effect of adenocarcinoma in situ are related to a better survival which could have an effect on the survival of adenocarcinomas. However this did not have a significant effect when comparing the original with the modified Norwegian cohort.
In the WHO classification of adenocarcinomas 1999 and 2004, the vast majority (94%) was of mixed subtype. This changed recently in resectable specimens by a multidisciplinary panel of lung cancer experts who developed a new classification in which BAC and mixed subtypes are replaced by adenocarcinoma in situ (AIS), and mixed subtypes are classified into five invasive subtypes estimated in 5% increments followed by identification and classification of the predominant histologic subtype (12). The relation between keratin expression and the new classification of adenocarcinomas has not been fully addressed in this article.
In conclusion, this study provides the first evidence that specific keratin patterns could play a major role in early stage NSCLC, and demonstrates promising results supporting its potential as a prognostic factor for survival. Examination of keratin patterns in early stage NSCLC may assist in selecting patients with poor prognoses for further adjuvant therapy and should be considered in future studies of early-stage NSCLC.
Supplementary material available online
Supplementary Tables I and Figures 1–2, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1049291
ionc_a_1049291_sm0725.pdf
Download PDF (1.5 MB)Acknowledgments
We thank L. Mortensen, O. Nielsen, and P. Mortensen all from the Department of Clinical Pathology Odense University hospital for excellent technical assistance, S. Svensson from The Danish Cancer Society, for generating high-quality tissue microarrays, and M. K. Occhipinti for editorial assistance. This work has been supported by grants from The Research Committee of Odense University Hospital, Denmark; The Danish Cancer Research Foundation; The Danish Cancer Society; The Cancer Foundation; and The Memorial Fund of Mette Hede Nielsen. The authors declare no conflicts of interests
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706–14.
- Novello S, Asamura H, Bazan J, Carbone D, Goldstraw P, Grunenwald D, et al. Early-stage lung cancer: 40s anniversary. J Thorac Oncol 2014;9:1434–42.
- Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le CT, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet 2010;375:1267–77.
- Moll R, Divo M, Langbein L. The human keratins: Biology and pathology. Histochem Cell Biol 2008;129:705–33.
- Broers JL, de LL, Rot MK, ter HA, Lane EB, Leigh IM, et al. Expression of intermediate filament proteins in fetal and adult human lung tissues. Differentiation 1989;40:119–28.
- Karantza V. Keratins in health and cancer: More than mere epithelial cell markers. Oncogene 2011;30:127–38.
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med 1998;157:2000–6.
- Mackinder MA, Evans CA, Chowdry J, Staton CA, Corfe BM. Alteration in composition of keratin intermediate filaments in a model of breast cancer progression and the potential to reverse hallmarks of metastasis. Cancer Biomark 2012;12:49–64.
- Seltmann K, Fritsch AW, Kas JA, Magin TM. Keratins significantly contribute to cell stiffness and impact invasive behavior. Proc Natl Acad Sci U S A 2013;110:18507–12.
- Hendrix MJ, Seftor EA, Chu YW, Trevor KT, Seftor RE. Role of intermediate filaments in migration, invasion and metastasis. Cancer Metastasis Rev 1996;15:507–25.
- Donnem T, Al-Shibli K, Andersen S, Al-Saad S, Busund LT, Bremnes RM. Combination of low vascular endothelial growth factor A (VEGF-A)/VEGF receptor 2 expression and high lymphocyte infiltration is a strong and independent favorable prognostic factor in patients with nonsmall cell lung cancer. Cancer 2010;116:4318–25.
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/American thoracic society/ European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6:244–85.
- Pohl M, Olsen KE, Holst R, Ditzel HJ, Hansen O. Tissue microarrays in non-small-cell lung cancer: Reliability of immunohistochemically-determined biomarkers. Clin Lung Cancer 2014;15:222–30.e3.
- Donnem T, Al-Saad S, Al-Shibli K, Delghandi MP, Persson M, et al. Inverse prognostic impact of angiogenic marker expression in tumor cells versus stromal cells in non small cell lung cancer. Clin Cancer Res 2007;13:6649–57.
- Camilo R, Capelozzi VL, Siqueira SA, Del Carlo BF. Expression of p63, keratin 5/6, keratin 7, and surfactant-A in non-small cell lung carcinomas. Hum Pathol 2006;37:542–6.
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005;97: 1180–4.
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982;31:11–24.
- Yamada A, Sasaki H, Aoyagi K, Sano M, Fujii S, Daiko H, et al. Expression of cytokeratin 7 predicts survival in stage I/IIA/IIB squamous cell carcinoma of the esophagus. Oncol Rep 2008;20:1021–7.
- Gharib TG, Chen G, Wang H, Huang CC, Prescott MS, Shedden K, et al. Proteomic analysis of cytokeratin isoforms uncovers association with survival in lung adenocarcinoma. Neoplasia 2002;4:440–8.
- Mehta R, Jain RK, Sneige N, Badve S, Resetkova E. Expression of high-molecular-weight cytokeratin (34betaE12) is an independent predictor of disease-free survival in patients with triple-negative tumours of the breast. J Clin Pathol 2010;63:744–7.
- Davis TL, Goldman AJ, Cress AE. Cell adhesion and cytoskeletal molecules in metastasis. In Cress AE, Nagle, RB, editors. Cancer metasis biology and treatment. The Netherlands: Springer Link; 2006.
- Bennouna J, Senellart H, Hiret S, Vaissiere N, Douillard JY. Impact of histology on survival of resected non-small cell lung cancer (NSCLC) receiving adjuvant chemotherapy: Subgroup analysis of the adjuvant vinorelbine (NVB) cisplatin (CDDP) versus observation in the ANITA trial. Lung Cancer 2011;74:30–4.
- Viberti L, Bongiovanni M, Croce S, Bussolati G. 34betaE12 cytokeratin immunodetection in the differential diagnosis of small cell tumors of lung. Int J Surg Pathol 2000;8: 317–22.
- Sturm N, Rossi G, Lantuejoul S, Laverriere MH, Papotti M, Brichon PY, et al. 34BetaE12 expression along the whole spectrum of neuroendocrine proliferations of the lung, from neuroendocrine cell hyperplasia to small cell carcinoma. Histopathology 2003;42:156–66.
- Donnem TP, Hald SM, Paulsen EE, Richardsen E, Al-Saad S, Kilvaer TK, et al. Stromal CD8 + T cell density – a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res Epub 2015 Feb 13.

