To the Editor,
Invasive lobular breast cancer (ILBrCa) accounts for only about 8–14% of a heterogeneous group of different histological types of breast cancer (BrCa), but it is still not infrequent due to high overall incidence of BrCa and an increasing rate of this type of tumor in older women since 1977 [Citation1]. BrCa has a well known glycolytic activity due to overexpression of glucose transporters [Citation2] that make F-18-fluoro- deoxyglucose (FDG) a suitable tracer, and BrCa one of the frequent indications for PET/CT [Citation3]. Significantly lower FDG uptake has been demonstrated in primary ILBrCa versus invasive ductal BrCa (IDBrCa) [Citation4,Citation5], with false negative rates of up to 66% for ILBrCa. A logical resulting extrapolation is that FDG uptake may be also low in metastasis of ILBrCa. This raises an important question regarding the relevance of FDG positron emission tomography/computerized tomography (PET/CT) for follow-up of ILBrCa. However, despite this common assumption, to the best of our knowledge, little clinical data has been reported to date on this issue.
In this study we set out to examine whether metastases of ILBrCa have lower FDG avidity similarly to the primary tumor, and thus whether FDG may be appropriate for systemic evaluation of patients with ILBrCa.
Patients and methods
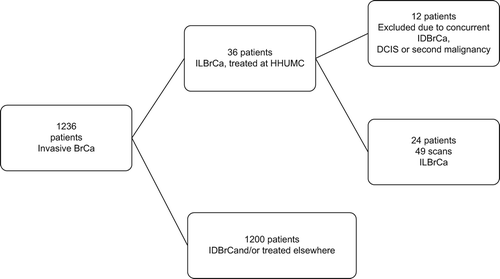
Patient selection is shown in the flowchart (). In brief, of 1236 PET/CT scans acquired at our institution of patients suffering from invasive BrCa, the sample was restricted to scans of patients with ILBrCa who underwent treatment at our institution to allow for follow-up and validation. Thirty-six patients fit these criteria, but 12 patients were subsequently excluded due to the presence of concurrent ductal carcinoma, including DCIS, or a secondary malignancy. Final sample included 49 FDG PET/CT scans on 24 patients (age: 47–79 years): eight patients with eight staging and seven follow-up studies, and 16 patients with 34 scans during follow-up only (total of eight staging and 41 follow-up studies). Ten patients underwent PET/CT once, six twice, six three times, and one patient each four and five times, respectively. Duration of follow-up ranged from 3 to 71 months (mean 38.9 ± 16.1, median 39 months); eight patients died during the follow-up period.
Kaplan-Meier survival analysis was performed.
PET/CT scans were acquired using a Discovery ST® PET/CT (GE Medical Systems, Milwaukee, WI, USA) with 18F-FDG produced at the hospital cyclotron. All the patients underwent a PET/CT scan in supine position after a six-hour fast, i.v. injection of 296.0–481.0 MBq of F-18-FDG, delay interval of 60–90 minutes between injection and scan.
All PET/CT scans were reviewed by a nuclear medicine and radiology physician (M.O.) and by an experienced nuclear medicine physician (M.K.). Detailed clinical information was traced by oncologists (E.T., B.U. and T.P.). PET/CT results were compared with bone scan, CT, magnetic resonance imaging (MRI) and with histo-pathological results, as available.
PET/CT scans were assessed by visual detection of foci and/or areas with increased uptake in comparison to background, further by semi-quantitative measurement (SUV max) and correlation with CT of the PET/CT. A false negative PET was recorded when active metastatic disease was proven by other modalities or clinical/biochemical evaluation but no corresponding pathological foci were seen on PET.
The protocol was approved by the Institutional Review Board according to principles of the Declaration of Helsinki.
Results
Twenty-four of 49 studies evaluated were positive while 25 were negative. In the 24 positive scans (four for staging and 20 for follow-up), per-lesion analysis showed that pathological findings were observed in 31 sites: bone (18), lymph nodes (LNs) axillary (3), retro-peritoneal and retro-crural (2), LN in hilum (1), pleura (2), mediastinum (1), liver (1), peritoneum (1), ovaries (1) and chest wall (1). Mean SUV max over all these lesions was 6.46 ± 3; median 6.25; range 2.2–15. Scan results for all patients are summarized in Supplementary Table I (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1054952).
In 16/18 pathological FDG foci in the skeleton, corresponding findings were seen on CT in form of three lytic (), five sclerotic and eight lytic-sclerotic lesions. In the two remaining bone foci, no corresponding anatomical changes were found on CT but clear correlation was found on bone scan. Bone scans were available in seven cases, and were positive in all, including the two CT negative findings. There was complete matching of PET, CT and bone scan findings in 5/7 cases.
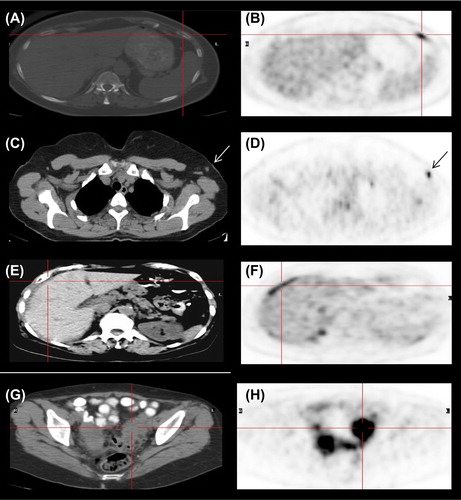
Among foci visualized in the lymph nodes on FDG PET, all three axillary findings (mean SUV max 6.85; range 3.7–10) were also visualized on CT as nodes of 0.8–1.0 cm (). Retroperitoneal and retro-crural LNs (SUV max 8.1 and 3.6, respectively) were also visualized on CT. Lung hilum LN was correlated with CT. Pleural effusion was seen in two patients (SUV max 2.2 and 3.6). In the first (Patient 13), PET/CT was performed eight months after a previous negative scan and showed disease progression with new pathological foci in the mediastinum and the pleura that were confirmed by biopsy. No pathological confirmation is available for the second case (Patient 23), due to subsequent death, but rapid disease progression essentially provides confirmation of the positive finding.
A pathological focus in the liver (Patient 9) was confirmed by a hypodense lesion of 1.5 cm on CT. Peritoneal spread () and metastases to ovary () were confirmed by biopsy. A chest wall collection indicating infection was seen in one patient.
True positive (TP) FDG PET were confirmed in 30/31 findings. The collection in the chest wall was the single false positive finding.
No pathological findings were found on other imaging modalities or on clinical follow-up on the 25 negative scans, confirming disease-free status. In one case (Patient 18), absence of disease in a CT-positive FDG-negative bone lesion was confirmed by biopsy. Likewise on the 24 positive scans, no additional pathological findings, not seen on PET, were detected, with only one exception of a lepto-meningeal spread (Patient 9).
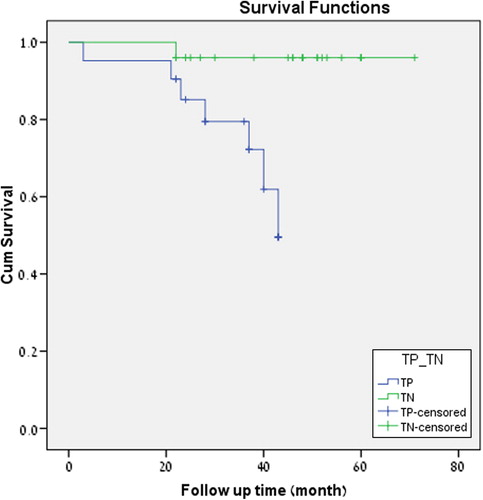
In summary, among 49 F-18-FDG PET/CT scans in 24 patients with histologically proven ILBrCa, 30/31 findings were TP. Negative PET/CTs were indeed true negative (TN) with the exception of one case of a lepto-meningeal spread. There was no clinical or imaging evidence for presence of additional metastases overlooked by PET, indicating a high sensitivity and specificity of PET/CT in the evaluation of metastases of ILBrCa. Kaplan-Meier survival analysis shows a significantly (p-value = 0.005) higher rate of death events in a cohort of TP studies than in TN scans ().
Discussion
In this study we examined FDG avidity of ILBrCa metastases in a group of 24 patients, and found that, contrary to common perception, FDG uptake was observed in sites of metastatic disease with only one exception, and subsequently confirmed by other imaging modalities, and that imaging and clinical follow-up did not reveal metastatic disease where FDG was negative. Furthermore, significant difference was seen in survival between FDG positive and negative groups.
Ueda et al. demonstrated a significantly lower local uptake of FDG by primary ILBrCa in comparison to IDBrCa [Citation5]. Avril et al. [Citation6,Citation7] as well as Buck et al. [Citation4] have also shown lower FDG avidity of primary ILBrCa.
Although both the primary tumor and metastases of ILBrCa tend to infiltrate rather than to build masses [Citation8], Hennipman et al. have shown higher activity of hexokinase and other glycolytic enzymes in metastases than in primary breast tumor [Citation9]. This finding could explain our results: with the exception of meningeal spread, all metastases of ILBrCa seen on other imaging modalities or revealed on extensive clinical follow-up were FDG-positive even though some of them demonstrated lower FDG accumulation than commonly seen in metastatic IDBrCa.
Furthermore, our findings are in keeping with the known predilection of metastases of ILBrCa to spread to peritoneal and retroperitoneal spaces; meningeal involvement and hepatic metastases are also more common [Citation8,Citation10]. Even in our small sample, there were cases with peritoneal, meningeal and hepatic metastases which are compatible with this clinical pattern of ILBrCa metastases as described by Winston et al. on CT [Citation11], thus supporting the identification of the FDG positive findings as ILBrCa metastases.
The main limitation of our work is the small population enrolled in this study with staging and follow-up cases analyzed together, due in part to the relatively low incidence of ILBrCa, and possibly also to few referrals due to the unproven assumption that FDG uptake is low or absent in metastatic ILBrCa. The difficulty of complete confirmation by biopsy, either in positive findings and particularly of the absence of disease is also a potential problem in any study of this type, and here correct evaluation and recognition of limitations are critical. Fortunately, other imaging modalities as well as disease-free clinical follow-up enabled us to confirm absence of disease at the time of PET/CT in most patients.
In summary, our study suggests that lower FDG uptake in primary tumor should not prevent systemic evaluation and follow-up by means of FDG PET in patients with ILBrCa. There is no clear evidence-based explanation for different FDG avidity of primary tumor and metastases, but as there is no histo-morphological difference between them, the most logical explanation is a difference in the activity of hexokinase and other glycolytic enzymes.
Conclusion
This study indicates that metastases of ILBrCa are FDG avid and that negative results correlate well with absence of metastatic disease. While further validation on a larger patient group is required, our results support use of F-18-FDG PET/CT for systemic evaluation and follow-up of patients with ILBrCa.
Supplementary material available online
Supplementary Table I available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1054952.
ionc_a_1054952_sm3640.pdf
Download PDF (29 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Li CI, Anderson BO, Porter P, Holt SK, Daling JR, Moe RE. Changing incidence rate of invasive lobular breast carcinoma among older women. Cancer 2000;88:2561–9.
- Brown RS, Wahl RL. Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer 1993;72:2979–85.
- Poeppel TD, Krause BJ, Heusner TA, Boy C, Bockisch A, Antoch G. PET/CT for the staging and follow-up of patients with malignancies. Eur J Radiol 2009;70:382–92.
- Buck A, Schirrmeister H, Kühn T, Shen C, Kalker T, Kotzerke J, et al. FDG uptake in breast cancer: Correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging 2002;29:1317–23.
- Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol 2008;38:250–8.
- Avril N1, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, et. al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med 2001;42:9–16.
- Avril N1, Rosé CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: Use and limitations. J Clin Oncol 2000;18:3495–502.
- Harris M, Howell A, Chrissohou M, Swindell RI, Hudson M, Sellwood RA. A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br J Cancer 1984;50:23–30.
- Hennipman A, Van Oirschot BA, Smits J, Rijksen G, Stacil GEJ. Heterogeneity of glycolytic enzyme activity and isozyme composition of pyruvate kinase in breast cancer. Tumour Biol 1988;9:178–88.
- Dixon AR, Ellis IO, Elston CW, Blamey RW. A comparison of the clinical metastatic patterns of invasive lobular and ductal carcinomas of the breast. Br J Cancer 1991;63:634–5.
- Winston CB, Hadar O, Teitcher JB, Caravelli JF, Sklarin NT, Panicek DM, et al. Metastatic lobular carcinoma of the breast: Patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am J Roentgenol 2000;175:795–800.