Abstract
Background. Treatment of postmolar gestational trophoblastic neoplasia (GTN) is often stratified according to FIGO score using methotrexate (MTX) for low-risk patients and first-line multi-agent chemotherapy (e.g. EMA-CO) for high-risk patients. In contrast, oral MTX may be given as first-line therapy to all GTN patients regardless of risk group. The aim was to examine the efficacy of oral MTX and a response-adapted treatment policy, which has been used for three decades at Aarhus University Hospital (AUH).
Material and methods. Seventy-one consecutive postmolar GTN patients treated 1981–2011 were included. Data were obtained from medical records, using histopathology and human choriogonadotropin (hCG) to verify the diagnosis. All patients received oral MTX as first-line chemotherapy. Second- and third-line chemotherapy was given according to response.
Results. Sixty-four (90%) patients were retrospectively categorized as FIGO low-risk disease, whereas seven patients (10%) had high-risk disease. Complete response to first-line oral MTX chemotherapy was observed in 35/71 (49%) patients, while 62/71 (87%) had complete remission on MTX (first-line) and/or MTX plus dactinomycin (second-line), without the use of multi-agent therapy. Nine patients (13%) received third-line multi-agent chemotherapy, six low-risk (67%) and three high-risk (33%) patients. There were no recurrences and no patients died as a consequence of toxicity or disease.
Conclusion. Fifty percent of all patients can be cured on oral MTX alone. By adding dactinomycin, about 90% are cured without use of multi-agent chemotherapy. The use of oral MTX as initial treatment can minimize the number of patients receiving multi-agent chemotherapy.
The medical treatment of gestational trophoblastic neoplasia (GTN) differs greatly around the world [Citation1,Citation2]. The reported primary remission rates following treatment with chemotherapy vary between 49–93% [Citation3], possibly reflecting differences in drug dosages, schedules, routes of administration, and selection of patients according to prognostic scoring systems [Citation4]. The modified WHO prognostic scoring system is currently the most widely used system dichotomizing patients into a low- and a high-risk group. For patients with low-risk GTN single-agent therapy with i.v. or i.m. methotrexate (MTX) or alternatively dactinomycin (Act-D) is recommended [Citation1,Citation3]. For patients with high-risk GTN multi-agent chemotherapy, such as EMA-CO (etoposide, MTX, Act-D, cyclophosphamide, vincristine) or EMA-EP (etoposide, MTX, Act-D, cisplatinum), is advocated [Citation2,Citation3,Citation5].
Despite these recommendations oral MTX for both low- and high-risk postmolar GTN patients has been used at Aarhus University Hospital (AUH) as primary chemotherapy to all patients for the past 30 years. Two studies including 15 and 37 patients treated with oral MTX supports this strategy reporting remission rates > 80% [Citation6,Citation7]. However, both these studies included non-metastatic patients only. The treatment response is closely monitored at AUH by measuring serum human chorionicgonadotropin (hCG) biweekly, and chemotherapy is intensified promptly if the response is considered unsatisfactory. The primary advantage of this strategy could be to evade multi-agent chemotherapy to high-risk patients, who can obtain remission on less intensive chemotherapy, thereby avoiding possible acute and long-term side effects of multi-agent chemotherapy [Citation8,Citation9]. Apart from direct toxic effect on several organs (e.g. cochlea, kidney, heart, lung) the long-term effects also include a non-trivial risk of second malignancies [Citation10]. In addition, intensive initial chemotherapy may cause fatal acute bleeding especially in cases with widespread metastases [Citation8,Citation11–13]. However, drug resistance, prolonged treatment time, and ultimately treatment failure are valid arguments against the use of single drug MTX as first-line treatment to all patients.
As GTN is a rare disease, these questions have so far been difficult to address. For the past three decades, the chemotherapeutic strategy and follow-up for patients with postmolar GTN has remained unchanged at AUH. The consistency to one treatment policy over such a long time period provides a unique possibility to obtain sufficient data for a valid retrospective data analysis. The primary objective of this study was therefore to examine the efficacy and safety of a response-adapted treatment strategy relying on oral MTX as first-line chemotherapy for postmolar GTN regardless of risk group.
Material and methods
This retrospective study included all patients treated with chemotherapy for postmolar GTN at AUH between January 1981 and September 2011. AUH is one of two national centers treating GTN, covering approximately 50% of the Danish population. We identified the study population by searching the AUH database, which incorporate all patients receiving chemotherapy for postmolar GTN. A total of 75 postmolar GTN patients were identified. Four patients were excluded; two low-risk patients treated initially with i.v. MTX and two patients who were treated for elevated hCG without histopathologic proof of disease. The study population thus consisted of 71 consecutive postmolar GTN patients treated with oral MTX as first-line treatment.
The diagnosis of postmolar GTN was based on histopathologic examinations of evacuates and on repetitive measurements of hCG. The indications for chemotherapy remained stable during the inclusion period and are now the basis for the National Danish guidelines (www.dgcg.dk) where hCG increase on at least two measurements after evacuation, persistent hCG plateau after re-evacuation and/or persistent vaginal bleeding are being used.
An expert gynecologic pathologist at AUH reviewed 69 of the 71 cases to verify the diagnosis of hydatidiform mole. In the remaining two patients, we were unable to retrieve material for histopathologic review. However, based on the information found in the original pathology reports from their primary hospitals, both patients were included in the study.
Fifty-eight patients (84%) had a history of a complete hydatidiform mole (CHM), whereas three patients (4%) were diagnosed with a partial hydatidiform mole (PHM). Eight patients (12%) had a hydatidiform mole, which could not be classified as either partial or complete by the pathologist.
Data on genetic constitution (karyotype) of the hydatidiform moles were obtained from the Danish Mole Project [Citation14]. In 38 (53%) of the patients with hydatidiform moles, genetic analyses were carried out and all had a diploid karyotype. All patients with diploid moles were also diagnosed as CHM by the expert pathologist.
Data on status at diagnosis, treatment, response and follow-up, were retrieved from the medical records. Pathology reports, imaging (chest x-ray, ultrasound scans of the uterus, and CT scans), and hCG levels were used to retrospectively classify the patients in low-risk (score < 6) and high-risk (score ≥ 7) groups according to the FIGO 2000 prognostic scoring and staging system [Citation15].
The chemotherapeutic protocols and follow-up regimes were unchanged throughout the study period (). First-line chemotherapy for all patients was oral MTX, administered every four weeks. Patients resistant to MTX, defined as an unsatisfactory half-life, increase, or stagnation of hCG, were offered either dose intensification (three-week MTX schedule) or second-line chemotherapy consisting of MTX combined with Act-D every four weeks (three-week schedule was used in a few patients). If the hCG levels did not normalize on second-line therapy, multi-agent chemotherapy (BEP) was used as third-line therapy. All patients received one additional course of the last used chemotherapy schedule after hCG normalization.
Table I. Chemotherapy schedules used at Aarhus University Hospital for the treatment of postmolar GTN since 1981.
Biochemical follow-up included monthly measurements of hCG for one year. No patients were lost to follow-up, and complete survival data was retrieved in the Central Person Register (CPR), providing unambiguous data on vital status. Patient files were also checked individually for non-fatal recurrences. A log-linear regression model was used to evaluate the correlation between hCG and treatment time. The study was approved and registered by the Danish Data Protection Agency.
Results
Sixty-four patients (90%) were retrospectively categorized as FIGO low-risk and seven patients (10%) as FIGO high-risk. Forty-nine patients (69%) had FIGO stage I disease, whereas 22 patients (31%) had metastatic disease (FIGO stage II–IV). Median FIGO scores for the patients cured on the different lines of chemotherapy are given in . Complete response to first-line oral MTX chemotherapy was achieved in 35/71 (49%), while 62/71 (87%) had complete response on first- or second-line therapy (MTX or MTX+ Act-D). Focusing on low-risk postmolar GTN we found that 58/64 (91%) were cured without use of third-line chemotherapy (BEP). In contrast, no high-risk patients were cured on first-line oral MTX, but 4/7 (57%) obtained complete remission already on second-line chemotherapy (MTX+ Act-D). Third-line chemotherapy (BEP) was used in 9/71 (13%) to finally obtain complete remission, i.e. six low-risk and three high-risk patients (). The median FIGO-score was 3 for the six low-risk patients and all but one had a tumor size larger than 3 cm.
Table II. Clinical characteristics of 71 patients treated for postmolar gestational trophoblastic neoplasia at Aarhus University Hospital in the period 1981–2011 distributed in three groups according to the line of chemotherapy on which they achieved complete remission. The number total of chemotherapy courses always include the course given for consolidation.
A total of 349 courses of chemotherapy were given, with a median of five courses per patient for complete response, including the one course given for consolidation (). Patients cured on first-line oral MTX had a median of three courses (range 1–10), corresponding to a median overall treatment time of 60 days (range 5–253). Patients cured on second-line chemotherapy had a median of six courses including three courses of MTX (range 1–5) before shift to second-line therapy. The nine patients cured on third-line chemotherapy had a median of eight courses (range 5–9) for remission, with a median overall treatment time of 162 days (range 107–227). In these patients a median of only one MTX course (range 1–5) was given before the shift to second-line therapy, and a median of two courses (range 1–4) on second-line chemotherapy before shifting to BEP.
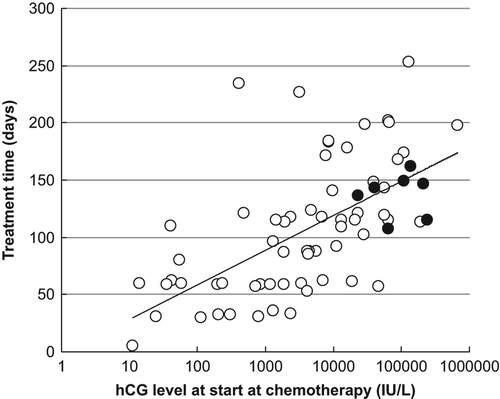
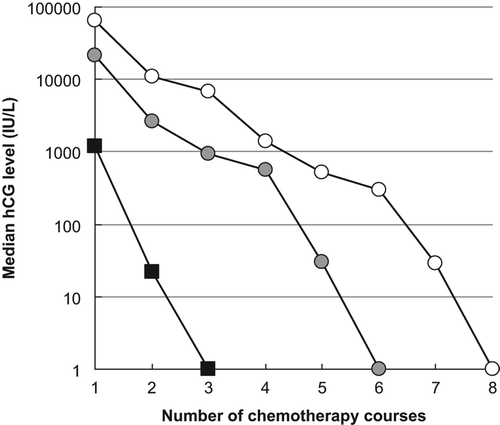
As demonstrated in , we found a LOG-linear correlation between the level of hCG at diagnosis and overall treatment time (R2 = 0.39; p < 0.001). The overall treatment time for high-risk patients (solid circles) was similar to the overall treatment time for low-risk patients for a given initial level of hCG. By calculating the median hCG level measured at the onset of each series of chemotherapy for the three groups of patients cured on the different lines of chemotherapy (), the impact of hCG and the risk of developing resistance to second- and third-line chemotherapy following oral MTX can be visualized. As the number of series of chemotherapy was linearly correlated with time (data not shown), the slope of the curves also indicates the decay of hCG (half-life). shows that the decay of hCG during the final phase of treatment was comparable for all three groups.
Due to toxicity two patients shifted from MTX to single agent Act-D and had complete remission on this. Likewise due to anemia and pulmonary symptoms four patients (44%) treated with BEP had bleomycin discontinued in the last course of chemotherapy, and for one patient the dose was reduced to 67% in the last two courses. Compared to patients treated with MTX and Act-D, side effects were reported more frequently in the group treated with BEP. In this group, four patients (44%) experienced cisplatin-induced ototoxicity. No fatal acute toxicities occurred and no recurrences were observed in any patient.
Discussion
In this study, the response-adapted treatment protocol for postmolar GTN used at AUH has been evaluated. To analyze the data in relation to the contemporary literature, the 71 patients with postmolar GTN were retrospectively classified in low- and high-risk groups. The retrospective design of the study may have had impact on FIGO scoring of the patients. The description of the imaging, e.g. tumor size and number of metastases was insufficient in 11 patients, which could have resulted in a lower FIGO score, as potential points received from imaging were not included. However, the relative distribution of patients into low- (90%) and high-risk (10%) groups were comparable to other published studies [Citation16] and most likely not more inaccurate than that reported in many prospective studies [Citation17].
The initial response rate obtained with oral MTX in this study (49.3%) was similar to another study using a comparable dose of i.m. MTX as initial chemotherapy [Citation18] but around 20% lower than reported in studies using higher doses of MTX, e.g. 125–200 mg i.v. or i.m. bi-weekly [Citation4,Citation16,Citation19,Citation20]. The inclusion of high-risk patients and the high percentage of metastatic patients (31%) could explain this finding [Citation4,Citation5,Citation16].
It has previously been observed that approximately 20–30% of all GTN patients may become resistant to the primary chemotherapeutic agent and require multi-agent chemotherapy [Citation2,Citation3,Citation20,Citation21]. In our study, the percentage of patients needing multi-agent chemotherapy was only 13% (N = 9). High hCG level, large tumor size and high FIGO score characterized these patients when compared to patients cured on oral MTX. Many studies therefore suggest that patients with hCG > 100 000 IU/L and FIGO score of 5–6 should be treated initially with multi-agent chemotherapy [Citation2,Citation17,Citation22,Citation23]. In the present study no high-risk patients and only 25% of the patients with FIGO score of 5–6 were cured on oral MTX. However, second-line chemotherapy (MTX combined with Act-D) cured 75% of all patients with a FIGO score 5–6 and 57% of the high-risk patients.
It could be argued that the adaptive treatment protocol used at AUH may prolong the overall treatment time with negative consequences for quality of life including postponement of any subsequent pregnancies. However, in our study the number of courses needed to achieve complete remission were fewer [Citation17,Citation18] and the total treatment time were comparable or even shorter than the treatment times reported by institutions using bi-weekly schedules [Citation22,Citation24]. The low number of MTX courses observed in patients shifted to second- or third-line chemotherapy indicates that all patients in the present study were carefully observed and promptly shifted to the next line of chemotherapy as soon as drug resistance was suspected. This demonstrates that the response-adapted protocol applied at AUH is feasible. However, this strategy requires high degree of compliance both on the part of the patient and from the involved healthcare personnel, which may not be feasible in all parts of the world.
We found that the overall treatment time was directly correlated to the hCG level at diagnosis regardless of risk group (). This suggests that the hCG level is by far the strongest prognostic factor and that the additional prognostic information obtained by the composite FIGO system is minimal. Further, the predictive information obtained from the repetitive hCG measurements during treatment () showed that the decay of hCG during the final phase of treatment was comparable for patients cured on first-, second- and third-line of chemotherapy. This finding suggests that resistance to MTX does not hamper the antineoplastic effect of the final treatment schedule that eventually leads to complete remission, which is in contrast to a previous report [Citation21]. Our data is therefore in agreement with other results showing that initial treatment with MTX has no detrimental impact on the chances for finally obtaining remission if drug resistance emerges and that multi-drug resistance occurs more frequently after failure of combination therapy than after failure of single-agent therapy [Citation12]. This also favors the protocol applied at AUH using low-dose single drug chemotherapy as initial therapy to all GTN patients, as the overall outcome is not affected, and multi-agent therapy is reserved if resistance appears.
In our study no relapses where found as opposed to studies reporting an overall recurrence rate of 3–8% [Citation2,Citation8,Citation16,Citation19]. Based on these observations, we had expected 3–5 cases of relapse, especially as we deliver only one course of consolidation chemotherapy (even in high risk) as opposed to three consolidation courses which has been recommended based on a large retrospective comparison of outcome in Dutch and British low risk GTN patients treated with MTX, where the risk of recurrence appeared to increase from 4% to 8% by decreasing the number of consolidation courses from three to two [Citation25]. However, differences in patient material, indications for chemotherapy, scoring systems and hCG assays hampers this UK–Dutch comparison. Another important difference is our adaptive approach which in this context implies that the consolidation course of chemotherapy is given with the drug combination (first-, second-, third-line) which has been found effective based on the response observed during treatment.
Only two patients (3%) had oral MTX replaced due to toxicity, both had reversible side effects (exanthema and raised liver enzymes) and were cured on Act-D. Toxicity, and especially hematological toxicity, as stated in other studies in relation to the use of MTX [Citation19,Citation26–28], was not reported, which could be due to the low dose of MTX and the four-weeks treatment interval.
In contrast, five of the nine patients (56%) receiving multi-agent chemotherapy had dose modifications due to side effects. As expected, ototoxicity (grade 2 and 3) was observed in patients treated with cisplatin.
No disease- or treatment-related deaths occurred in the 30-year period, which we believe is connected to the low-dose of initial chemotherapy used in our study. This view is substantiated by the occurrence of early deaths reported in studies using multi-agent as initially chemotherapy in high-risk patients with large tumor burden [Citation8,Citation11–13]. With the adaptive protocol used at AUH all patients may be spared from this potential danger apparently without risking chemotherapy resistance, prolongation of overall treatment time or treatment failure. The adaptive strategy also spares some (high-risk) patients from the toxicities of multi-agent chemotherapy compared to the setting where first-line treatment with multi-agent chemotherapy is based on risk grouping at diagnosis.
In conclusion, this study therefore questions the dogma that all high-risk patients should be treated initially with multi-agent chemotherapy. In fact, this retrospective study points to response-adapted chemotherapy rather than initial risk group stratification as a better way to maximize the therapeutic window. From the patient perspective, most patients would also prefer low-dose chemotherapy, as long as it does not compromise the treatment efficacy [Citation24].
Acknowledgements
This study was supported by scholarship from The Danish Cancer Society and a Scholarship for the World Congress XVII of the International Society for the Study of Trophoblastic Diseases, Chicago, 2013.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alazzam M, Tidy J, Hancock BW, Osborne R. First line chemotherapy in low risk gestational trophoblastic neoplasia. Cochrane Database Syst Rev 2009;1:CD007102.
- Goldstein DP, Berkowitz RS. Current management of gestational trophoblastic neoplasia. Hematol Oncol Clin North Am 2012;26:111–31.
- Lurain JR. Gestational trophoblastic disease II: Classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol 2011;204:11–8.
- Chapman-Davis E, Hoekstra AV, Rademaker AW, Schink JC, Lurain JR. Treatment of nonmetastatic and metastatic low-risk gestational trophoblastic neoplasia: Factors associated with resistance to single-agent methotrexate chemotherapy. Gynecol Oncol 2012;125:572–5.
- El-Helw LM, Hancock BW. Treatment of metastatic gestational trophoblastic neoplasia. Lancet Oncol 2007;8: 715–24.
- Barter JF, Soong SJ, Hatch KD, Orr JW, Jr, Partridge EC, Austin JM, Jr, et al. Treatment of nonmetastatic gestational trophoblastic disease with sequential intramuscular and oral methotrexate. Gynecol Oncol 1989;33:82–4.
- Barter JF, Soong SJ, Hatch KD, Orr JW, Jr, Partridge EC, Austin JM, Jr, et al. Treatment of nonmetastatic gestational trophoblastic disease with oral methotrexate. Am J Obstet Gynecol 1987;157:1166–8.
- Lybol C, Thomas CM, Blanken EA, Sweep FC, Verheijen RH, Westermann AM, et al. Comparing cisplatin-based combination chemotherapy with EMA/CO chemotherapy for the treatment of high risk gestational trophoblastic neoplasia. Eur J Cancer 2013;49:860–7.
- Deng L, Zhang J, Wu T, Lawrie TA. Combination chemotherapy for primary treatment of high-risk gestational trophoblastic tumour. Cochrane Database Syst Rev 2013; 1:CD005196.
- Rustin GJ, Newlands ES, Lutz JM, Holden L, Bagshawe KD, Hiscox JG, et al. Combination but not single-agent methotrexate chemotherapy for gestational trophoblastic tumors increases the incidence of second tumors. J Clin Oncol 1996;14:2769–73.
- Hoogland HJ, Arends JW, Blijham GH, Braat SH, de Haan J, van Krimpen C. Death from chemotherapy in gestational trophoblastic disease. Eur J Obstet Gynecol Reprod Biol 1988;29:167–72.
- Bower M, Newlands ES, Holden L, Short D, Brock C, Rustin GJ, et al. EMA/CO for high-risk gestational trophoblastic tumors: Results from a cohort of 272 patients. J Clin Oncol 1997;15:2636–43.
- Alifrangis C, Agarwal R, Short D, Fisher RA, Sebire NJ, Harvey R, et al. EMA/CO for high-risk gestational trophoblastic neoplasia: Good outcomes with induction low-dose etoposide-cisplatin and genetic analysis. J Clin Oncol 2013;31:280–6.
- Niemann I, Hansen ES, Sunde L. The risk of persistent trophoblastic disease after hydatidiform mole classified by morphology and ploidy. Gynecol Oncol 2007;104: 411–5.
- FIGO Oncology Committee. FIGO staging for gestational trophoblastic neoplasia 2000. FIGO Oncology Committee. Int J Gynaecol Obstet 2002;77:285–7.
- Sita-Lumsden A, Short D, Lindsay I, Sebire NJ, Adjogatse D, Seckl MJ, et al. Treatment outcomes for 618 women with gestational trophoblastic tumours following a molar pregnancy at the Charing Cross Hospital, 2000–2009. Br J Cancer 2012;107:1810–4.
- Osborne RJ, Filiaci V, Schink JC, Mannel RS, Alvarez Secord A, Kelley JL, et al. Phase III trial of weekly methotrexate or pulsed dactinomycin for low-risk gestational trophoblastic neoplasia: A gynecologic oncology group study. J Clin Oncol 2011;29:825–31.
- Gilani MM, Yarandi F, Eftekhar Z, Hanjani P. Comparison of pulse methotrexate and pulse dactinomycin in the treatment of low-risk gestational trophoblastic neoplasia. Aust N Z J Obstet Gynaecol 2005;45:161–4.
- Khan F, Everard J, Ahmed S, Coleman RE, Aitken M, Hancock BW. Low-risk persistent gestational trophoblastic disease treated with low-dose methotrexate: Efficacy, acute and long-term effects. Br J Cancer 2003;89:2197–201.
- McNeish IA, Strickland S, Holden L, Rustin GJ, Foskett M, Seckl MJ, et al. Low-risk persistent gestational trophoblastic disease: Outcome after initial treatment with low-dose methotrexate and folinic acid from 1992 to 2000. J Clin Oncol 2002;20:1838–44.
- Lu WG, Ye F, Shen YM, Fu YF, Chen HZ, Wan XY, et al. EMA-CO chemotherapy for high-risk gestational trophoblastic neoplasia: A clinical analysis of 54 patients. Int J Gynecol Cancer 2008;18:357–62.
- Khoo SK, Sidhu M, Baartz D, Yip WL, Tripcony L. Persistence and malignant sequelae of gestational trophoblastic disease: Clinical presentation, diagnosis, treatment and outcome. Aust N Z J Obstet Gynaecol 2010;50:81–6.
- El-Helw LM, Coleman RE, Everard JE, Tidy JA, Horsman JM, Elkhenini HF, et al. Impact of the revised FIGO/WHO system on the management of patients with gestational trophoblastic neoplasia. Gynecol Oncol 2009; 113:306–11.
- McGrath S, Short D, Harvey R, Schmid P, Savage PM, Seckl MJ. The management and outcome of women with post-hydatidiform mole ‘low-risk’ gestational trophoblastic neoplasia, but hCG levels in excess of 100 000 IU l(-1). Br J Cancer 2010;102:810–4.
- Lybol C, Sweep FC, Harvey R, Mitchell H, Short D, Thomas CM, et al. Relapse rates after two versus three consolidation courses of methotrexate in the treatment of low-risk gestational trophoblastic neoplasia. Gynecol Oncol 2012;125:576–9.
- Eiriksson L, Wells T, Steed H, Schepansky A, Capstick V, Hoskins P, et al. Combined methotrexate-dactinomycin: An effective therapy for low-risk gestational trophoblastic neoplasia. Gynecol Oncol 2012;124:553–7.
- Abrao RA, de Andrade JM, Tiezzi DG, Marana HR, Candido dos Reis FJ, Clagnan WS. Treatment for low-risk gestational trophoblastic disease: Comparison of single-agent methotrexate, dactinomycin and combination regimens. Gynecol Oncol 2008;108:149–53.
- Soper JT, Clarke-Pearson DL, Berchuck A, Rodriguez G, Hammond CB. 5-Day methotrexate for women with metastatic gestational trophoblastic disease. Gynecol Oncol 1994;54:76–9.

