ABSTRACT
Background. The purpose of the study was to assess dose and time dependence of radiotherapy (RT)-induced changes in regional lung function measured with single photon emission computed tomography (SPECT) of the lung and relate these changes to the symptomatic endpoint of radiation pneumonitis (RP) in patients treated for non-small cell lung cancer (NSCLC).
Material and methods. NSCLC patients scheduled to receive curative RT of minimum 60 Gy were included prospectively in the study. Lung perfusion SPECT/CT was performed before and three months after RT. Reconstructed SPECT/CT data were registered to treatment planning CT. Dose to the lung was segmented into regions corresponding to 0–5, 6–20, 21–40, 41–60 and > 60 Gy. Changes (%) in regional lung perfusion before and after RT were correlated with regional dose and symptomatic RP (CTC grade 2–5) outcome.
Results. A total of 58 patients were included, of which 45 had three-month follow-up SPECT/CT scans. Analysis showed a statistically significant dose-dependent reduction in regional perfusion at three-month follow-up. The largest population composite perfusion loss was in 41–60 Gy (42.2%) and > 60 Gy (41.7%) dose bins. Lung regions receiving low dose of 0–5 Gy and 6–20 Gy had corresponding perfusion increase (-7.2% and -6.1%, respectively). Regional perfusion reduction was different in patients with and without RP with the largest difference in 21–40 Gy bin (p = 0.02), while for other bins the difference did not reach statistical significance. The risk of symptomatic RP was higher for the patients with perfusion reduction after RT (p = 0.02), with the relative risk estimate of 3.6 (95% CI 1.1–12).
Conclusion. Perfusion lung function changes in a dose-dependent manner after RT. The severity of radiation-induced lung symptoms is significantly correlated with SPECT perfusion changes. Perfusion reduction early after RT is associated with a high risk of later development of symptomatic RP.
With advances in radiotherapy (RT) techniques radiation is distributed non-uniformly throughout the different regions of the lung. We have shown lung function assessed by perfusion imaging single photon emission computed tomography (SPECT) before RT to be heterogeneously distributed in the lung with different degrees of perfusion defects in the lung [Citation1]. In previous studies, regional changes in SPECT perfusion have been related to regional radiation dose [Citation2–6]. It has also been established that RT-induced reductions in regional lung perfusion are related to changes in pulmonary function tests (PFT) after RT [Citation6,Citation7]. PFT represent an objective global lung function measure. However, some studies have proven PFT to be a weak predictor of radiation-induced pulmonary dysfunction, because the changes in PFT do not always correlate well with patients’ clinically experienced toxicity [Citation8–11]. The association between perfusion changes in the lung assessed by SPECT and clinically scored radiation pneumonitis (RP) has not been established. Moreover, the underlying radiation sensitivity of lung tissue is not completely understood [Citation12–14]. We hypothesise that regional changes in the lung perfusion on SPECT three months after curative chemo-radiotherapy are dose-dependant. The purpose of the study was to correlate changes in SPECT perfusion over time with radiation dose, and to examine, whether lung perfusion changes early after RT can predict the risk of development of symptomatic RP.
Material and methods
Between 2012 and 2013 a total of 74 consecutive patients with histologically verified non-small cell lung cancer (NSCLC) undergoing curative RT were included prospectively. Three patients did not meet eligibility criteria (histologically verified NSCLC, minimum RT dose of 60 Gy, absence of other uncontrolled malignancies) and were excluded. Of 71 eligible patients, 13 were treated with stereotactic body radiation therapy (SBRT) technique with high dose per fraction, and therefore were excluded from the analysis.
To measure the lung perfusion, SPECT/CT was performed after i.v. administration of 99mTc as previously described [Citation1]. SPECT/CT scans were performed within a week before RT, as well as at three months after the end of RT. A total RT dose of 60–66 Gy was delivered in 2-Gy fractions with an intensity-modulated radiation therapy (IMRT) technique.
Data analysis
As shown in Supplementary Figure 1 (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1061695), regional dose bins corresponding to 0–5, 6–20, 21–40, 41–60 and > 60 Gy were created on treatment planning CT. The CT of the SPECT/CT was registered to the treatment planning CT using rigid and deformable registration in MIM Software (MIM version 6.4). Dose distribution from the treatment planning CT was correlated to the map of SPECT perfusion within the healthy lung tissue [total lung minus gross tumour volume (GTV)]. The regional perfusion reduction (RPR) at each dose bin was calculated as described in details in the Supplementary Appendix (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1061695). To assess the overall population's RPR in each dose bin, individual patient's perfusion changes were combined using weighted average technique adjusting for volume irradiated at each dose level [Citation2,Citation5], as described in details in the Appendix.
Pulmonary morbidity was scored by the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) version 4 [Citation15]. Pneumonitis symptoms and radiographic changes on CT were scored on CTCAE scale grade 0–5. RP was defined as radiation-induced symptoms (dyspnoea, cough, pain and low-grade fever) and radiographic changes on CT. Patients with evidence of tumour recurrence or thoracic disease progression (PD) during follow-up were excluded from the study. The patients were divided into two groups: patients with no RP symptoms and radiological changes only (CTC grade 0–1, non-RP group), and patients with symptomatic to severe RP (CTC grade 2–5, RP group).
Statistics
A paired samples t-test was used to compare SPECT perfusion change with time (three-month post-RT relative to pre-RT). Student's t-test was used to compare mean RPR in different dose bins for the patients in RP versus non-RP groups. The Kaplan-Meier method was used to calculate time to symptomatic RP (grade 2–5) from the end of RT. Patients who did not develop RP were censored at the last available follow-up, disease progression or death. The log-rank test was used to analyse differences in time to RP in patients with perfusion reduction or increase in the whole lung. Two-tailed p-values less than 0.05 were considered statistically significant.
This single centre prospective study was approved by the Ethical Committee of The Central Denmark Region (study ID number 1-10-72-11-12). Written informed consent was obtained from each patient upon inclusion.
Results
Of 58 eligible patients with pre-treatment SPECT/CT, 45 had SPECT/CT performed at three-month follow-up (median 3.2 months range 2.1–4.2 months), and were analysed in the present study. Patient characteristics are listed in . Drop out after RT completion was due to disease progression (N = 7), general weakness without detectable PD (N = 5), as well as one death of RP. Characteristics of the patients with SPECT at baseline and three-month follow-up are presented in . Dose dependent population's composite perfusion loss on SPECT is shown in . Negative perfusion loss in the low dose bins (0–5 and 6–20 Gy) indicate improvement in perfusion, while perfusion loss is progressively increasing with dose in the higher dose bins of 21–40, 41–60 and over 60 Gy. Perfusion increase with time in 0–5 Gy dose bin was statistically significant (p = 0.04). The reduction in perfusion with time in 21–40, 41–60, > 60 Gy dose bins was also statistically significant (p-values < 0.01 for all three bins).
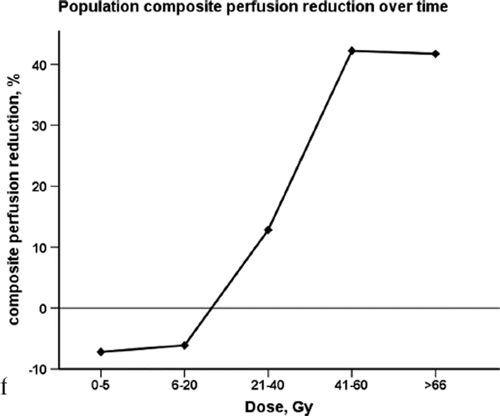
Table I. Patient characteristics (n = 45).
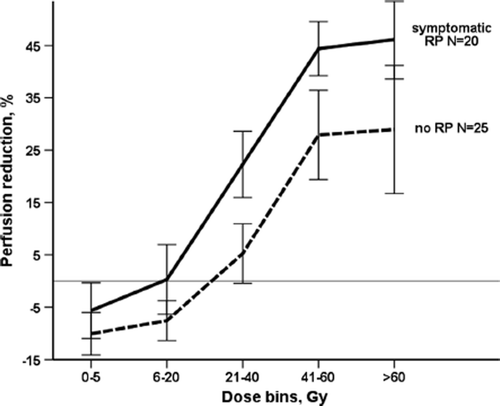
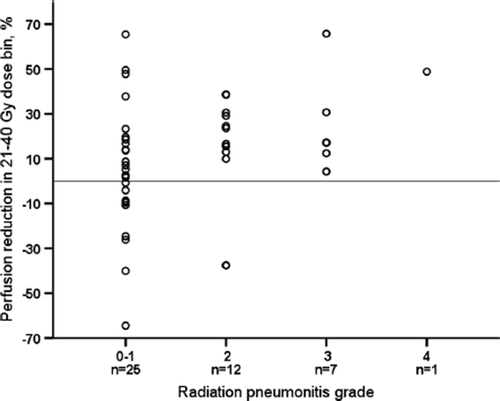
RPR was different in patients with and without RP, as presented in . The difference was largest in 21–40 Gy bin (p = 0.02). For the other bins the difference did not reach statistical significance. For the whole lung regardless of the dose received, RPR was -3% in patients with no RP, and 7% in RP group (p = 0.03). In the correlation analysis on , RPR in the 21–40 Gy dose bin showed statistically significant, though modest correlation with RP grade (Spearman's r = 0.4, p = 0.02).
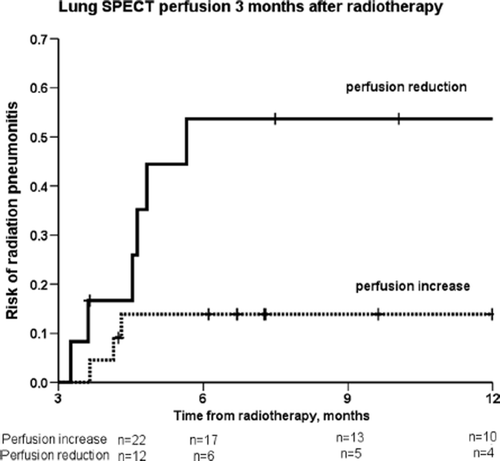
Median time to symptomatic RP was 2.7 months (range 0.8–5). We compared the risk of RP for patients with and without reduction in perfusion by calculating time from the end of RT to the development of symptomatic RP. Only patients who did not develop symptomatic RP at three months of follow-up were included in the actuarial analysis. As presented on the risk of symptomatic RP was higher for the patients with perfusion reduction after RT (p = 0.02). The relative risk estimate for the patients with perfusion reduction was 3.6 (95% CI 1.1–12).
Discussion
This study demonstrates that perfusion reduction in the lung after RT measured with SPECT happens in a dose-dependent manner, with a small function increase in low dose areas and function reduction up to 42% in high dose areas. This is possibly due to function being shunted to the areas receiving low dose. This is the first study that demonstrates the relationship between perfusion reduction assessed by SPECT/CT and the severity of radiation-induced symptoms in patients treated with IMRT. Patients with perfusion reduction had a high risk of later developing symptomatic RP. The advantage of the study is the prospective design with close follow-up of symptomatic and imaging endpoints. Deformable registration of the pre- and post-treatment scans with accuracy within few mm was used in the study. The advantage of deformable registration to be more accurate for comparing the changes after RT has been previously shown by Palma et al. [Citation16]. The limitation of our study is patients’ drop-out in follow-up, which reduced the amount of SPECT/CT scans available for the analysis.
Effects of normalisation of dose data to lower doses of 0–5 Gy have been discussed previously [Citation2,Citation17]. The threshold to which normalisation should be performed is also debatable. In this study we have not performed normalisation of dose data, as it limits interpretations of the results for low dose bins. On the contrary, unnormalised data are able to suggest a threshold for RT injury, where dose-response curve slope crosses x-axis. In our study, it corresponds to the 6–20 Gy dose bin (), and thus reveals a negative RPR in the low dose bins. The possible mechanism can be that perfusion is being shunted to the low dose areas as a compensation for the regional lung damage in the high dose areas. Moreover, in the study of Woel et al. population analyses were repeated for unnormalised data, as well as normalised with outcomes largely unchanged with time progression of perfusion injury restricted to high-dose regions [Citation2].
The results in our study showing dose-dependent RT-induced regional lung dysfunction are in agreement with previous studies [Citation5,Citation18]. Woel et al. additionally showed that RT-induced reductions in regional perfusion progress with time, with most of this injury manifest within 12 months after RT [Citation2]. Few authors have related changes in SPECT perfusion with symptomatic outcomes with no association found in patients irradiated for different thoracic tumours [Citation9,Citation19]. Other studies have focused on relating perfusion changes to more objective endpoint-changes in PFT [Citation7,Citation17,Citation20–22], and yielded highly significant results. In comparison to the aforementioned studies, we investigated perfusion and symptomatic pulmonary injury in the settings of modern technological advances, such as combined SPECT/CT imaging [Citation4,Citation23] and radiotherapy technique (IMRT, SBRT) [Citation24,Citation25]. Our patient cohort is homogeneous with NSCLC only and high RT dose of ≥ 60 Gy.
The toxicity scoring of radiation-induced pulmonary morbidity in our study is not relying on treatment indication alone, but rather on the combination of symptoms’ severity, radiographic changes and intervention indicated, as described in CTCAE v.4 for pneumonitis. The present study supports the assumption that development of radiation-induced lung symptoms may reflect the sum of regional injury plus compensatory increases in function in the low dose areas. The compensatory ability to shunt the function to these areas is most likely impaired in the patients with symptomatic RP.
Only patients with symptomatic RP at three months or later after RT were included in the actuarial analysis (). For these patients the risk of later development of RP according to the perfusion changes on three-month SPECT could be assessed. Therefore, an introduction of SPECT/CT into a follow-up routine three months after RT completion can help to identify the patients who are at high risk of developing RP. Closer monitoring and early treatment may help these patients not to develop severe symptoms [Citation26]. Performing SPECT/CT at three-month follow-up and quantifying perfusion loss at this time point may also improve the diagnostics of RP for the individual patient. As perfusion loss correlates with the grade of RP in our study, it may be a valuable tool for RP diagnostics, compared to PFT, which does not show the same correlation [Citation1]. The finding of significant perfusion loss in 21–40 Gy dose range in symptomatic RP patients can be used in the future studies as dose constraints. Thus, SPECT-based treatment planning may allow sparing the lung volume receiving 20 Gy and higher. RP is an acute toxicity developing few weeks to several months after RT [Citation27]. In our patient cohort symptomatic RP happened three months after RT (median time 2.7 months). There were 11 patients who developed symptomatic RP before the three-month follow-up SPECT scan was performed. To assess the risk of RP for the whole patient cohort, analysis of one-month follow-up SPECT is on the way.
In conclusion, this study demonstrates that changes in perfusion on SPECT three months after RT are dose-dependent with lung function reduction up to 42% in high dose areas and correlate with severity of radiation toxicity symptoms. Furthermore, perfusion reduction early after RT is associated with a high risk of later development of symptomatic RP.
Supplementary material available online
Supplementary Appendix and Figure 1 available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1061695
ionc_a_1061695_sm3728.pdf
Download PDF (1.8 MB)Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Farr KP, Kramer S, Khalil AA, Morsing A, Grau C. Role of perfusion SPECT in prediction and measurement of pulmonary complications after radiotherapy for lung cancer. Eur J Nucl Med Mol Imaging 2015;42:1315–1324.
- Woel RT, Munley MT, Hollis D, Fan M, Bentel G, Anscher MS, et al. The time course of radiation therapy- induced reductions in regional perfusion: A prospective study with > 5 years of follow-up. Int J Radiat Oncol Biol Phys 2002;52:58–67.
- Scheenstra AE, Rossi MM, Belderbos JS, Damen EM, Lebesque JV, Sonke JJ. Local dose-effect relations for lung perfusion post stereotactic body radiotherapy. Radiother Oncol 2013;107:398–402.
- Partridge M, Yamamoto T, Grau C, Hoyer M, Muren LP. Imaging of normal lung, liver and parotid gland function for radiotherapy. Acta Oncol 2010;49:997–1011.
- Marks LB, Munley MT, Spencer DP, Sherouse GW, Bentel GC, Hoppenworth J, et al. Quantification of radiation- induced regional lung injury with perfusion imaging. Int J Radiat Oncol Biol Phys 1997;38:399–409.
- De Jaeger K, Seppenwoolde Y, Boersma LJ, Muller SH, Baas P, Belderbos JS, et al. Pulmonary function following high-dose radiotherapy of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2003;55:1331–40.
- Fan M, Marks LB, Hollis D, Bentel GG, Anscher MS, Sibley G, et al. Can we predict radiation-induced changes in pulmonary function based on the sum of predicted regional dysfunction? J Clin Oncol 2001;19:543–50.
- Abratt RP, Willcox PA, Smith JA. Lung cancer in patients with borderline lung functions – zonal lung perfusion scans at presentation and lung function after high dose irradiation. Radiother Oncol 1990;19:317–22.
- Marks LB, Fan M, Clough R, Munley M, Bentel G, Coleman RE, et al. Radiation-induced pulmonary injury: Symptomatic versus subclinical endpoints. Int J Radiat Biol 2000;76:469–75.
- Wang J, Cao J, Yuan S, Ji W, Arenberg D, Dai J, et al. Poor baseline pulmonary function may not increase the risk of radiation-induced lung toxicity. Int J Radiat Oncol Biol Phys 2013;85:798–804.
- Dang J, Li G, Ma L, Diao R, Zang S, Han C, et al. Predictors of grade >/ = 2 and grade >/ = 3 radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with three-dimensional conformal radiotherapy. Acta Oncol 2013;52:1175–80.
- Farr KP, Khalil AA, Knap MM, Moller DS, Grau C. Development of radiation pneumopathy and generalised radiological changes after radiotherapy are independent negative prognostic factors for survival in non-small cell lung cancer patients. Radiother Oncol 2013;107:382–8.
- Grau C, Hoyer M, Alber M, Overgaard J, Lindegaard JC, Muren LP. Biology-guided adaptive radiotherapy (BiGART) – more than a vision? Acta Oncol 2013;52:1243–7.
- Tucker SL, Liao Z, Dinh J, Bian SX, Mohan R, Martel MK, et al. Is there an impact of heart exposure on the incidence of radiation pneumonitis? Analysis of data from a large clinical cohort. Acta Oncol 2014;53:590–6.
- National Cancer Institute. Common terminology criteria for adverse events: CTCAE Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [cited 2015 April 11].
- Palma DA, van Sornsen de Koste JR, Verbakel WF, Senan S. A new approach to quantifying lung damage after stereotactic body radiation therapy. Acta Oncol 2011;50:509–17.
- Garipagaoglu M, Munley MT, Hollis D, Poulson JM, Bentel GC, Sibley G, et al. The effect of patient-specific factors on radiation-induced regional lung injury. Int J Radiat Oncol Biol Phys 1999;45:331–8.
- Boersma LJ, Damen EM, de Boer RW, Muller SH, Valdes Olmos RA, Hoefnagel CA, et al. A new method to determine dose-effect relations for local lung-function changes using correlated SPECT and CT data. Radiother Oncol 1993;29:110–6.
- Abratt RP, Willcox PA, Smith JA. Lung cancer in patients with borderline lung functions – zonal lung perfusion scans at presentation and lung function after high dose irradiation. Radiother Oncol 1990;19:317–22.
- Ma J, Zhang J, Zhou S, Hubbs JL, Foltz RJ, Hollis DR, et al. Association between RT-induced changes in lung tissue density and global lung function. Int J Radiat Oncol Biol Phys 2009;74:781–9.
- Theuws JC, Muller SH, Seppenwoolde Y, Kwa SL, Boersma LJ, Hart GA, et al. Effect of radiotherapy and chemotherapy on pulmonary function after treatment for breast cancer and lymphoma: A follow-up study. J Clin Oncol 1999;17:3091–100.
- Marks LB. Physiology-based studies of radiation-induced normal tissue injury. Radiother Oncol 1999;51:101–3.
- Hutton BF. Recent advances in iterative reconstruction for clinical SPECT/PET and CT. Acta Oncol 2011;50: 851–8.
- Moller DS, Khalil AA, Knap MM, Hoffmann L. Adaptive radiotherapy of lung cancer patients with pleural effusion or atelectasis. Radiother Oncol 2014;110:517–22.
- Lavrenkov K, Singh S, Christian JA, Partridge M, Nioutsikou E, Cook G, et al. Effective avoidance of a functional SPECT-perfused lung using intensity modulated radiotherapy (IMRT) for non-small cell lung cancer (NSCLC): An update of a planning study. Radiother Oncol 2009;91: 349–52.
- Williams JP, Johnston CJ, Finkelstein JN. Treatment for radiation-induced pulmonary late effects: Spoiled for choice or looking in the wrong direction? Curr Drug Targets 2010;11:1386–94.
- Appelt AL, Vogelius IR, Farr KP, Khalil AA, Bentzen SM. Towards individualized dose constraints: Adjusting the QUANTEC radiation pneumonitis model for clinical risk factors. Acta Oncol 2014;53:605–12.