ABSTRACT
Background. Adaptive strategy with daily online tumour match is a treatment option when treating locally advanced lung cancer patients with curative intended radiotherapy (RT).
Material and methods. Fifty-two consecutive lung cancer patients treated with soft tissue match, adaptive RT and small planning target volumes (PTV) margins were analysed. A control group of 52 consecutive patients treated with bone match, no adaptive strategy and larger margins was included. Patients were followed with computed tomography (CT) scans every third month. CT-images showing loco-regional recurrences were identified. The recurrence gross tumour volume was delineated and registered with the original radiation treatment plan to identify site of failure. All patients were toxicity-scored using CTCAE 4.03 grading scale. Data were analysed using the Kaplan-Meier analysis.
Results. The median follow-up time was 16 months (3–35). Within a year, 35% of the patients in the adaptive group (ART-group) and 53% in the control group (No-ART-group) experienced loco-regional failure, showing improved loco-regional control in the ART group (p = 0.05). One patient in the ART-group and four patients in the No-ART-group showed marginal failure. Median overall progression-free survival time for the ART-group was 10 months (95% CI 8–12), and 8 months (95% CI 6–9) for the No-ART-group. Severe pneumonitis (grade 3–5) decreased from 22% in the No-ART-group to 18% in the ART-group (non-significant, p = 0.6). No significant difference in severe dysphagia was found between the two groups.
Conclusion. In the first small cohort of patients investigated, implementation of soft-tissue tumour match and adaptive strategies for locally advanced lung cancer patients increased the loco-regional control rate without increasing treatment-related toxicity.
One of the major challenges when treating lung cancer patients with radiotherapy (RT) is to find the balance between tumour control and treatment toxicity. Tumour control could be increased by escalating the radiation dose [Citation1–3]. Meanwhile, escalating the radiation dose to the tumour is limited by the toxic effect of radiation on healthy lung tissue [Citation4]. Increasing the treatment precision by implementing soft tissue match [Citation5] and adaptive radiotherapy (ART) [Citation6–8] may reduce the treatment volume and hence potentially decrease the irradiation of healthy lung tissue. This is highly important since several studies have shown a correlation between increasing mean lung dose (MLD) and the risk of developing radiation pneumonitis (RP) [Citation9–11]. However, implementing smaller treatment volumes due to the high precision of ART introduces the potential risk of marginal failures as large margins incidentally may cover microscopic disease not included in the target delineated.
During the course of RT, patients experience relocation between tumour and lymph nodes [Citation12,Citation13]. Additionally, changes during the treatment due to atelectasis, pleural effusion and/or pneumonia/pneumonitis may cause variation in lung density as well as dramatic anatomical changes [Citation14]. By using the strategy of ART, changes above a certain threshold is acted on by re-scanning and re-optimisation of the treatment plan [Citation6].
ART based on a soft tissue tumour match was recently implemented for patients with locally advanced lung cancer in our institution. As a consequence of the implementation, the treatment margins were reduced. In this study, we analyse if patterns of loco-regional failures have changed due to the introduction of ART and soft tissue tumour match. Additionally, we evaluate if the introduction of smaller treatment volumes has resulted in a decrease in RP.
Material and methods
Patients
An ART-group with 54 consecutive lung cancer patients treated after April 2013 was compared to a control group (No-ART-group) with 55 consecutive lung cancer patients treated from January through October 2012. The group was matched on pathological diagnosis. Patients undergoing RT post-operatively because of microscopic disease were excluded (two in the ART-group and three in the No-ART-group). All patients underwent pre-treatment staging with diagnostic 18FDG PET and computed tomography (CT) scanning, fine needle aspiration of the tumour and endoscopic bronchial ultrasound aspiration of the lymph nodes. All tumours were pathologically proven.
All patients received curative intended chemo-radiotherapy. The patients with non-small cell lung cancer (NSCLC) received cisplatin/carboplatin and vinorelbine concomitant or sequential with 60–66 Gy in 30–33 fractions (F), 5F/week, while patients with small-cell lung cancer (SCLC) received cisplatin/carboplatin and etoposide concomitant or sequential with 45 Gy in 30 F, 10 F/week. Patient- and tumour-related characteristics are seen in . Four patients had stage IV disease. These patients had cerebral (n = 3) or subcutane (n = 1) metastasis and all of them received radical resection (n = 3) or stereotactic treatment (n = 1) of the metastasis.
Table I. Patients- and tumour characteristics.
From bone match to tumour match and ART
All patients underwent a free-breathing 18FDG positron emission tomography (PET)-CT planning scan with 3 mm slice spacing and pixel size of 1 × 1 mm2 for the CT scan. The patients were positioned with both arms above the head in a standard or an individualised immobilisation device. The CT scan was performed with intra-venous contrast as a time- resolved four-dimensional (4D) scan and the mid-ventilation phase was selected for delineation of normal tissue and for treatment planning. The gross tumour volumes of the tumour (GTV-T) and the lymph nodes, GTV-N, were delineated using the maximum intensity projection thus accounting for respiratory tumour movement [Citation15].
All patients were treated with intensity-modulated radiation therapy (IMRT) and identical normal tissue constraints were used. In both groups, a daily cone beam computed tomography (CBCT) scan was acquired and used for set up. In the No-ART-group, the patients were set-up on the thoracic vertebrae in the target area and planned with large margins. The ART-patients were set-up on the primary tumour with smaller margins. The margins applied in the two groups accounting for microscopic disease and intrafractional breathing motion are summarised in . Additionally, planning target volume margins (PTV) accounting for delineation uncertainties, target deformations, inter- and intrafractional baseline shifts, deviations in MLC, couch and CBCT iso-centre position, CT-distortion and partial volume effects are quoted. In the ART group, interfractional base line shifts are only applicable for the lymph node site due to set-up on the primary tumour yielding a very small margin for the primary tumour.
Table II. Margins and dosimetric characteristics.
The adaptive strategy, shown in Supplementary Figure 1 (available online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2015.1062544) was based on a daily online evaluation by the radiation therapists (RTTs), who evaluated if the position of the tumour, the lymph nodes and the vertebral column was within pre-set tolerances. Furthermore, the RTTs noted appearance/disappearance of atelectasis, pleural effusion or pneumonia. If any change above the tolerance was seen online for three consecutive fractions, a medical physicist evaluated if the patient would benefit from a re-scanning and re-planning. In the ART group, 12 patients were re-planned due to the described evaluations. In the No-ART-group no systematic re-planning took place but five patients were re-planned due to large changes found accidentally by the RTTs. Only the bony anatomy match was evaluated systematically in this group.
When patients were re-planned a 4DCT scan was acquired. Target GTV-T and GTV-N were delineated on the updated CT scan based on both a rigid and a daemons-based deformable transfer from the planning-CT, followed by manual correction by an experienced radiation oncologist. The intention was to keep the volume of the target unchanged, even if noticeable shrinkage had occurred. A new clinical target volume (CTV) was created by adding a 5 mm margin to the GTV. Normal tissue structures were corrected based on deformable transfer to the new CT scan.
Pattern of failure
Patients were followed every third month with CT scans and clinical evaluation during the first year and afterwards every sixth month for the next five years or until either disease progression or death.
CT scans were used to identify site of failure and all intra-thoracic failures were delineated with a GTV of the recurrence (GTV-REC). The CT scan of the recurrence was afterwards rigidly registered with the original radiation treatment planning CT scan by using Smart Adapt version 11 (Varian Medical Systems, Palo Alto, CA, USA). All CT scans were primarily matched on the vertebral column and each match was inspected. In some patients, clear anatomical deviations of the chest organs were observed. For these patients, either the trachea or the bronchi was used for matching. An experienced radiation oncologist evaluated all the fused CT images. Loco-regional recurrences were classified as being located either in- or outside the planned GTV-T or GTV-N. Furthermore, recurrences lying outside GTV-T and/or GTV-N were evaluated with regards to the vicinity to the 95% isodose line of the treatment plan. Marginal failure was defined as recurrence within 2 cm of the 95% isodose line [Citation16]. Distant metastases were defined as progression outside the thorax, in the contralateral lung or haematogenous spread in both lungs. All recurrences were verified by biopsy.
Evaluation of toxicity
All patients were toxicity-scored with regards to dysphagia, dyspnoea and cough by using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Fifty-six patients were scored prospectively during clinical visits while 48 patients were scored retrospectively by reviewing medical records. The toxicity scoring took place at clinical visits before, during and after the RT-course. The scoring continued until the patient developed progressive disease in thorax or died.
Furthermore, patients in both the ART- and the No-ART-group had their medical records from any hospital in the region checked to detect admissions possibly related to RT. The toxicity scoring regarding dyspnoea and cough, in addition to CT scans and notes from admissions at any hospital in the region, was used to score the grade of RP using the CTCAE 4.03 grading scale. Patients with grade 3 or higher were considered to have severe RP. The diagnosis of severe RP was made if the patients were admitted with severe symptoms (dyspnoea, dry cough, low grade fever), had radiographic evidence of radiation-induced interstitial lung damage and if no other obvious cause was found for the symptoms of the patients (pleural effusion, cardiac disease or lung infection).
Statistical analysis
Data were analysed using SPSS statistical software version 20. All analyses were calculated from the first day of RT. When calculating incidences of severe RP, patients were censored from analysis when they had recurrent disease within the thorax (local failure, regional failure, lung or pleural metastasis) or died. Student t-tests were used to compare mean dosimetric characteristics (MLD, GTV and PTV). The Kaplan-Meier method was used to calculate time- to-severe-RP, loco-regional-failure, progression-free survival and overall-survival. The log-rank test was used to analyse statistical significance. A p-value of less than 0.05 was considered statistically significant.
Results
Median follow-up time in the No-ART-group was 17 months (range 3–35 months). Median follow-up time for the ART-group was 16 months (range 3–20 months).
In , all patients- and tumour-characteristics are similar for the two groups, except for the distribution of gender (p = 0.04). There was a significant difference in both PTV and MLD between the two groups as a result of the smaller margins applied in the ART-group (see ).
Fifty patients developed loco-regional failure. Of these, 32 occurred in the No-ART-group while 18 occurred in the ART-group. The incidence of loco-regional failure at 12-months was 53% for the No-ART-group and 35% for the ART-group () (p = 0.05). Twelve months was chosen as a cut point to make sure that follow-up was equal in the two groups. After these 12 months, 12 patients in the No-ART-group and six patients in the ART-group developed recurrence in T-site only. Five patients in the No-ART-group and seven patients in the ART-group developed N-site failures only, while nine patients in the No-ART-group and three patients in the ART-group had recurrence simultaneously in both T- and N-site. The distribution of loco-regional failures after 12 months for each group is shown in .
Figure 1. Loco-regional progression in lung cancer patients treated with curative chemo-radiotherapy with an adaptive strategy (ART-group) and without an adaptive strategy (No-ART-group). A) Actuarial analysis of the time to loco-regional progression compared for the two groups. B) The 12-months distribution of loco-regional failures in tumour site (T-site), lymph node site (N-site) or both is exposed for the two groups.
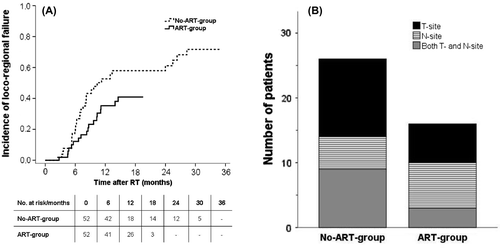
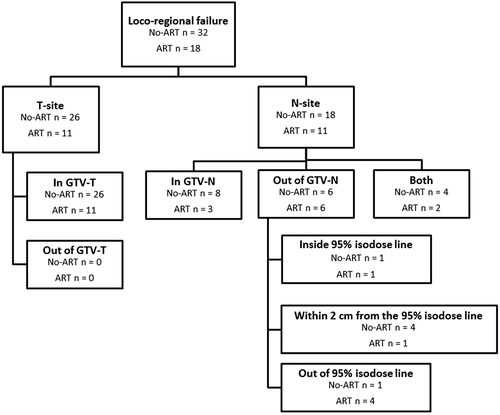
An analysis of all loco-regional failures occurring during follow-up is shown in . Thirty-seven patients showed failure at the T-site while 29 patients showed failure at the N-site. Patients who developed recurrence in T- and N-site simultaneously were analysed separately. All failures in T-site occurred in the planning GTV-T for both groups, while the relapse in N-site occurred outside the GTV-N for 12 patients. Of these patients, only four patients in the No-ART- and one patient in the ART-group showed marginal failure. These five marginal failures were located outside the GTV-N as defined on the planning CT but within 2 cm from the 95% isodose line. An example of a marginal failure is shown in the Supplementary Figure 2 (available online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2015.1062544) which represents recurrence in a lymph node station not included in the primary target definition of GTV-N. Thus, it is not a result of geographical miss. This was similar in all five cases of marginal failure. Four patients in the No-ART-group and two patients in the ART-group had recurrence both in- and outside the planned GTV-N. These patients had multiple metastases in several lymph nodes both in- and outside the mediastinum.
Figure 2. Analysis of all loco-regional failures occurring in lung cancer patients (n = 104) treated with radiotherapy with an adaptive strategy (ART) or without an adaptive strategy (No-ART). Failures were analysed with regards to GTV-T and GTV-N as delineated on the planning CT. Recurrences lying outside GTV-T and/or GTV-N were evaluated with regards to the vicinity to the 95% isodose line of the treatment plan. This was done to detect incidences of marginal failure, defined as failure within 2 cm of the 95% isodose line. Patients who developed recurrence in T- and N-site simultaneously were analysed separately on T- and N-site.
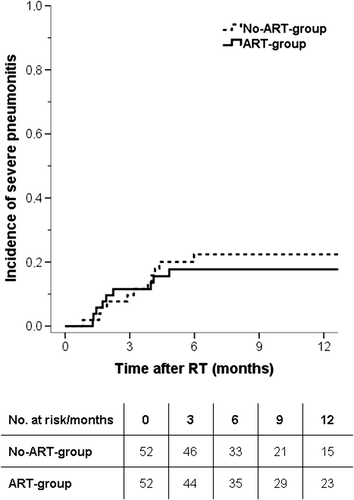
In the No-ART-group, 11 of 52 patients developed severe RP while nine of 52 patients developed severe RP in the ART-group. The incidence of severe pneumonitis at six months was 22% in the No-ART-group and 18% in the ART-group (p = 0.6) (). One patient developed a grade 5 pneumonitis. This patient was diagnosed with SCLC and originated from the ART-group. The patient had severe comorbidity with follicular lymphoma and chronic obstructive pulmonary disease.
Figure 3. Incidences of severe pneumonitis (grade ≥ 3) in lung cancer patients treated with curative chemo-radiotherapy with an adaptive strategy (ART-group) and without an adaptive strategy (No-ART-group).
Median time to severe dysphagia was 24 days (range 13–176 days). No statistically significant difference in incidences of severe dysphagia (grade ≥ 3) was found between the two groups.
Median progression-free survival time for the ART-group was 10 months (95% CI 8–12 months), and 8 months (95% CI 6–9 months) for the No-ART-group. Median overall survival for all patients was 18 months (95% CI 16–21 months).
Discussion
The present study investigated clinical outcome of the first small cohort of lung cancer patients treated with RT based on ART and soft tissue tumour match. The results demonstrate that the implementation of ART has improved loco-regional control. Additionally, no increase in the incidences of marginal failure was observed.
Using ART for lung cancer treatment is a quite new approach. Though many studies have suggested the benefit of ART, none have studied its clinical implications on the outcome. Hunter et al. [Citation17] suggested that modern image guidance may improve outcome for patients who experienced loco-regional failure peripherally or marginally. By using ART, our study did actually find an overall increase in the loco-regional control.
Inadequate dose to the target volume, due to anatomical and geometrical changes during the RT course, may explain the significantly higher occurrence rate of loco-regional failures in the No-ART-group although five patients were re-planned once in this group due to accidentally observed deviations. The number of patients re-planned was substantially higher in the ART-group and furthermore, the re-planning was based on a systematic approach where patients were re-planned up to three times during the treatment course. Another reason, explaining the difference in loco-regional control, could be that only bone match was performed in the No-ART-group. Van Elmpt et al. [Citation18] have shown, by acquiring CT scans during the treatment course, that for bone match, 31% of the patients showed displacements larger than 5 mm of the tumour. Thus, for some patients in the No-ART-group, only part of the tumour might have received the prescribed dose. Additionally, in the No-ART-group no systematic evaluation of anatomical changes as, e.g. disappearance of atelectasis was performed. In a cohort of 163 patients with lung cancer, Møller et al. [Citation14] showed that 12% of the patients would benefit from an adaptive strategy to avoid inadequate dose to the target resulting from anatomical changes. In the present study, ART was combined with daily soft tissue tumour match, securing sufficient dose coverage for all treatment fractions. This is consistent with the findings of Yeung et al. [Citation5], who showed that daily CBCT soft-tissue registration on tumour, without concurrent ART, minimized the daily setup errors and allowed a safe reduction in the PTV margins.
We have reported an increased loco-regional control by using ART. However, it remains a great challenge to improve outcome for locally advanced lung cancer patients treated with RT. A retrospective study by Schytte et al. [Citation19] reported that most loco-regional failures consist of solitary intrapulmonary failures (73%). This is in agreement with the findings in the present study, where the largest over-all failure proportion was observed at the tumour site. However, in the present study, a higher incidence of loco-regional failures was reported compared to other studies [Citation19–21]. Increased loco-regional control has been directly related to dose-escalation [Citation1–3]. Partridge et al. [Citation22] have demonstrated a clear dose-response relationship for NSCLC patients treated with radical RT, while Nielsen et al. [Citation23] demonstrated the possibility of introducing inhomogeneous dose escalation in order to enhance loco-regional control. The RTOG-0617-study by Bradley et al. [Citation21] clearly stated that dose escalation could have a fatal outcome. However, the usage of both ART and soft tissue tumour match combined with a decrease in treatment margins could potentially be safe allowing dose escalation without increasing fatal toxicity.
The marginal failures reported in this study were only seen in lymph nodes and not in tumours. Of five marginal failures, none were related to geographical miss. Instead, the marginal failures in this study could be comprehended as a result of decisions made at the time of target definition.
The mean PTV decreased by 33% in the ART group resulting in a significant decrease in the MLD by 1.8 Gy. We expected that the decrease in MLD would reduce the incidences of RP. MLD has been reported to be a significant predictor of severe pneumonitis [Citation9,Citation24]. Giuliani et al. [Citation10] showed that increased MLD was associated with grade ≥ 3 RP in RT of SCLC patients, regardless of fractionation. The present study showed a trend towards decrease in the incidences of severe pneumonitis. In order to obtain a statistically significant difference between the two groups, many more patients should be included due to the low incidence of RP grade ≥ 3. A review by Rodrigues et al. [Citation25] reported that RP usually develops in 13–37% of patients receiving radical RT. In comparison, the incidence of RP of 18% in the ART-group and 22% in the No-ART-group is rather low. This may be related to a low MLD in our study.
The prospective and retrospective mix of toxicity scoring is a limitation of this study. However, this is dealt with by using grade ≥ 3 RP as an endpoint which, by our definition, requires hospitalisation. This measurement is quite objective since no admission at any hospital in Denmark would have happened without being noted in the medical files of the patients. These files were all read during the toxicity scoring of every patient and the diagnosis of severe pneumonitis was used only if every other possible diagnosis was excluded. By adding an evaluation of radiation-induced lung changes on CT scans to the diagnosis of pneumonitis, this current study tries to make the scoring of RP as accurate as possible.
In conclusion, the first small cohort of locally advanced lung cancer patients treated by using RT based on daily soft tissue tumour match and an adaptive strategy displays improved loco-regional control without increasing treatment-related toxicity. Furthermore, PTV and hence MLD are significantly reduced. The reduction in the PTV-margins seems to be safe without any increase in marginal failure.
Supplementary material available online
Supplementary Figures 1 and 2 available online at http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2015.1062544.
ionc_a_1062544_sm3724.pdf
Download PDF (537 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kong F-M, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: Long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005;63:324–33.
- Machtay M, Bae K, Movsas B, Paulus R, Gore EM, Komaki R, et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: An analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2012;82:425–34.
- Kim JO, Chu KP, Fairchild A, Ghosh S, Butts C, Chu Q, et al. Dose-escalated hypofractionated intensity-modulated radiation therapy with concurrent chemotherapy for inoperable or unresectable non-small cell lung cancer. Am J Clin Oncol Epub 2014 Oct 20.
- Bradley JD, Moughan J, Graham MV, Byhardt R, Govindan R, Fowler J, et al. A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: Phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys 2010;77:367–72.
- Yeung AR, Li JG, Shi W, Newlin HE, Chvetsov A, Liu C, et al. Tumor localization using cone-beam CT reduces setup margins in conventionally fractionated radiotherapy for lung tumors. Int J Radiat Oncol Biol Phys 2009;74:1100–7.
- Yan D, Vicini F, Wong J, Martinez A. Adaptive radiation therapy. Phys Med Biol 1997;42:123–32.
- Sonke J-J, Belderbos J. Adaptive radiotherapy for lung cancer. Semin Radiat Oncol 2010;20:94–106.
- Woodford C, Yartsev S, Dar AR, Bauman G, Van Dyk J. Adaptive radiotherapy planning on decreasing gross tumor volumes as seen on megavoltage computed tomography images. Int J Radiat Oncol Biol Phys 2007;69:1316–22.
- Dang J, Li G, Ma L, Diao R, Zang S, Han C, et al. Predictors of grade ≥ 2 and grade ≥ 3 radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with three-dimensional conformal radiotherapy. Acta Oncol 2013;52:1175–80.
- Giuliani ME, Lindsay PE, Kwan JYY, Sun A, Bezjak A, Le LW, et al. Correlation of dosimetric and clinical factors with the development of esophagitis and radiation pneumonitis in patients with limited-stage small-cell lung carcinoma. Clin Lung Cancer 2015;16:216–20.
- Appelt AL, Vogelius IR, Farr KP, Khalil A, Bentzen SM. Towards individualized dose constraints: Adjusting the QUANTEC radiation pneumonitis model for clinical risk factors. Acta Oncol 2014;53:605–12.
- Knap MM, Hoffmann L, Nordsmark M, Vestergaard A. Daily cone-beam computed tomography used to determine tumour shrinkage and localisation in lung cancer patients. Acta Oncol 2010;49:1077–84.
- Schmidt ML, Hoffmann L, Kandi M, Møller DS, Poulsen PR. Dosimetric impact of respiratory motion, interfraction baseline shifts, and anatomical changes in radiotherapy of non-small cell lung cancer. Acta Oncol 2013;52:1490–6.
- Møller DS, Khalil AA, Knap MM, Hoffmann L. Adaptive radiotherapy of lung cancer patients with pleural effusion or atelectasis. Radiother Oncol 2014;110:517–22.
- Ezhil M, Vedam S, Balter P, Choi B, Mirkovic D, Starkschall G, et al. Determination of patient-specific internal gross tumor volumes for lung cancer using four-dimensional computed tomography. Radiat Oncol 2009;4:4.
- Rajpara RS, Schreibmann E, Fox T, Stapleford LJ, Beitler JJ, Curran WJ, et al. Locoregional tumor failure after definitive radiation for patients with stage III non-small cell lung cancer. Radiat Oncol 2014;9:187.
- Hunter KU, Kong F-MS, Chetty IJ, Cronin P, Tatro D, Marn C, et al. Pattern of failure after high-dose thoracic radiation for non-small cell lung cancer: The University of Michigan experience. J Radiat Oncol 2012;1:267–72.
- van Elmpt W, Ollers M, van Herwijnen H, den Holder L, Vercoulen L, Wouters M, et al. Volume or position changes of primary lung tumor during (chemo-)radiotherapy cannot be used as a surrogate for mediastinal lymph node changes: The case for optimal mediastinal lymph node imaging during radiotherapy. Int J Radiat Oncol Biol Phys 2011;79:89–95.
- Schytte T, Nielsen TB, Brink C, Hansen O. Pattern of loco-regional failure after definitive radiotherapy for non-small cell lung cancer. Acta Oncol 2014;53:336–41.
- Jeppesen SS, Schytte T, Jensen HR, Brink C, Hansen O. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: An updated retrospective study on local failure and survival rates. Acta Oncol 2013;52:1552–8.
- Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187–99.
- Partridge M, Ramos M, Sardaro A, Brada M. Dose escalation for non-small cell lung cancer: Analysis and modelling of published literature. Radiother Oncol 2011;99: 6–11.
- Nielsen TB, Hansen O, Schytte T, Brink C. Inhomogeneous dose escalation increases expected local control for NSCLC patients with lymph node involvement without increased mean lung dose. Acta Oncol 2014;53:119–25.
- Farr KP, Khalil AA, Knap MM, Møller DS, Grau C. Development of radiation pneumopathy and generalised radiological changes after radiotherapy are independent negative prognostic factors for survival in non-small cell lung cancer patients. Radiother Oncol 2013;107:382–8.
- Rodrigues G, Lock M, D’Souza D, Yu E, Van Dyk J. Prediction of radiation pneumonitis by dose-volume histogram parameters in lung cancer – a systematic review. Radiother Oncol 2004;71:127–38.

