ABSTRACT
Background. The aim of the present study was to compare the biological effectiveness of carbon ions relative to x-rays between tumor control, acute skin reaction and late RIF of CDF1 mice.
Material and methods. CDF1 mice with a C3H mouse mammary carcinoma implanted subcutaneously on the foot of the right hind limb were irradiated with single fractions of either photons, or 12C ions using a 30-mm spread-out Bragg peak. The endpoint of the study was local control (no tumor recurrence within 90 days). For the acute skin reaction, non-tumor bearing CDF1 mice were irradiated with a comparable radiation scheme, and monitored for acute skin damage between Day 7 and 40. Late RIF was assessed in the irradiated mice.
Results. The TCD50 (dose producing tumor control in 50% of mice) values with 95% confidence interval were 29.7 (25.4–34.8) Gy for C ions and 43.9 (39.2–49.2) Gy for photons, with a corresponding Relative biological effectiveness (RBE) value of 1.48 (1.28–1.72). For acute skin damage the MDD50 (dose to produce moist desquamation in 50% of mice) values with 95% confidence interval were 26.3 (23.0–30.1) Gy for C ions and 35.8 (32.9–39.0) Gy for photons, resulting in a RBE of 1.36 (1.20–1.54). For late radiation-induced fibrosis the FD50 (dose to produce severe fibrosis in 50% of mice) values with 95% confidence interval were 26.5 (23.1–30.3) Gy for carbon ions and 39.8 (37.8–41.8) Gy for photons, with a RBE of 1.50 (1.33–1.69).
Conclusion. The observed RBE values were very similar for tumor response, acute skin damage and late RIF when irradiated with large doses of high- linear energy transfer (LET) carbon ions. This study adds information to the variation in biological effectiveness in different tumor and normal tissue models.
Particle beams can be focused more selectively on the tumor, effectively reducing unwanted dose to healthy tissue and achieving higher conformal dose distributions. Furthermore, different radiation qualities can be selected, such as low- and high linear energy transfer (LET); proton and carbon ions, respectively [Citation1,Citation2]. High-LET radiation produces dense energy deposits, leading to a markedly increased efficiency of cell killing [Citation2]. The concept of relative biological effectiveness (RBE) has been introduced to account for this increased effectiveness. RBE is defined as the ratio of a dose from photons to a dose from any other particle to produce the same biological effect [Citation3]. This is complicated by the fact that RBE is not a constant, but depends on factors such as the specific tissue, endpoint, fraction size, particle energy, cell cycle distribution, and the tumor microenvironment [Citation1,Citation3]. Higher RBE not only increases tumor control probability (TCP), but also leads to higher normal tissue complication. Several in vitro experiments have carefully assessed the RBE of carbon ion beams for a large range of cell lines, and models such as the Local Effect Model [Citation4] were developed in order to predict the RBE for arbitrary mixed radiation fields in various tissue types. The efficacy of both protons and 12C carbon ions has been investigated in clinical settings for certain tumor types [Citation5–14], however, the discussion remains even on a non-clinical level as to what is the efficacy of carbon ions. Due to the RBE increasing for decreasing dose, the gain in biological effect between peak and plateau has been discussed [Citation15]. A recent modeling study of the therapeutic ratio [local control rates (TCP) compared to the probability of normal tissue complications (NTCP)], demonstrated that the peak-to-entrance ratio for different dose levels, tissue types, and field configurations points towards that a clear gain is expected using ions carbon or helium ions compared to protons [Citation16]. The different aspects of carbon ion therapy are very thoroughly covered in more reviews [Citation17–19].
In previous in vivo experiments assessing the effect of carbon ion radiation on tumor response, only a limited number of studies focused on tumor control [Citation20–22]. To elucidate the biological variation in radiation response, more in vivo studies are needed. The aim of the present study was to compare the biological effectiveness of carbon ions relative to x-rays using tumor control, acute skin reaction and late radiation-induced fibrosis of CDF1 mice as endpoint.
Material and methods
Animal and tumor models
All experiments were performed on 10–14-week-old female CDF1 mice. The tumor model used was the C3H/Tif mouse mammary carcinoma. Its derivation and maintenance has been described previously [Citation23]. Experimental tumors were produced following sterile dissection of large flank tumors. Macroscopically viable tumor tissue was minced with a pair of scissors, and 5–10 μl of this material were injected into the foot of the right hind limb of the experimental animals. This location ensured easy access to the tumor for treatment without involvement of critical normal tissue in the treatment field. Treatments were carried out when tumors had reached a tumor volume of about 200 mm3, which generally occurred within three weeks after inoculation. All experiments were performed under the Institutional and National Guidelines for Animal Welfare.
Tumor irradiation and response
The procedure for radiation treatment has previously been described [Citation24]. All treatments to tumor bearing feet were administered to non-anesthetized mice placed in a Lucite jig. Their tumor-bearing legs were exposed and loosely attached to the jig with tape, without impairing the blood supply to the foot. To secure homogeneity of the radiation dose for the x-ray irradiation, tumors were immersed in a water bath (25°C) with 7.1 cm of water and 9 mm PMMA (phantom entrance window) between the radiation source and the target. Seven dose groups (10–42 Gy for carbon ions and 38–68 Gy for photons) with six animals in each were irradiated; in total 84 animals for tumor response.
The expected response was based on the known response for x-rays for this tumor model [Citation24,Citation25], and previously reported RBE values for tumor control [Citation20,Citation21], with adequate margins to ensure a full dose response curve. As the uncertainty in the expected RBE gave a large span for where the dose response curve could be expected, the carbon ion doses were placed with 5 Gy intervals.
The response of tumors to treatment was assessed using tumor growth and a local tumor control assay. For the growth studies, tumor volume was determined on a daily basis using the formula D1*D2*D3*π/6 (where the D values represents the three orthogonal diameters).
For tumor control, mice were followed at weekly intervals up to 90 days post-treatment. Response was calculated as the percentage of animals in each treatment group showing no recurrent tumor at 90 days after treatment.
Of the 84 irradiated mice in the tumor control experiment, two mice had to be euthanized due to general health issues, and have been excluded from the analysis. Of the remaining mice, one mouse displayed a very atypical tumor regrowth with metastasis in the neck at Day 63 after treatment. The tumor and the metastasis were sent to pathologic inspection, which showed that the tumor was similar to the original C3H tumors, and was scored as a tumor regrowth.
Skin damage
Irradiations were performed locally on normal foot skin as described for the tumor experiments. However, due to the absence of a tumor, it was not possible to loosely attach the leg to the restraining jig with tape. Fixing of the leg in the correct position for treatment was therefore achieved by applying a small drop of histoacrylic glue to the restraining jig in the region of the uppermost part of the leg. The leg was then firmly compressed against the jig with tape for a 5-minute period, after which time the tape was loosened and left for 10 minutes prior to any treatment. Only the right hind leg was irradiated. Seven dose groups (10–40 Gy for carbon ions and 26–46 Gy for photons) with six animals in each were irradiated; in total 84 animals for normal tissue response.
The expected response was based on the known response for x-rays for this mouse strain [Citation24,Citation25], and previously reported RBE values for early response of skin reaction [Citation26,Citation27]. As with the tumor control assay, due to the uncertainty in the expected RBE of carbon ions, doses were placed with 5 Gy intervals to ensure a full dose response curve.
After treatment the leg was easily detached from the jig. For scoring the skin damage, a previous published skin score table [Citation28] was used, as described in [Citation24]. The skin damage is scored in seven steps of 0.5, with 3.5 being the maximum value. Mice were observed on a daily basis between seven and 40 days following treatment, and the percentage of animals in each treatment group with a score of 3.5 (moist desquamation) was determined.
Radiation-induced fibrosis
Late radiation-induced fibrosis (RIF) was assessed in the irradiated mice, using a modification of the leg contracture model described by Stone [Citation29]. Both non-tumor bearing mice and tumor bearing mice without tumor regrowth were included in the analysis. Based on the degree of extensibility of the irradiated leg, the endpoint for RIF was defined as a permanent reduction in extensibility of at least 75% relative to the untreated leg. This endpoint was considered to correspond to severe irreversible subcutaneous fibrosis. The difference in extensibility of the irradiated legs was measured every second week from Day 49 to at least Day 322. For the x-ray curve, previous published data was included in the analysis [Citation30].
Dose delivery
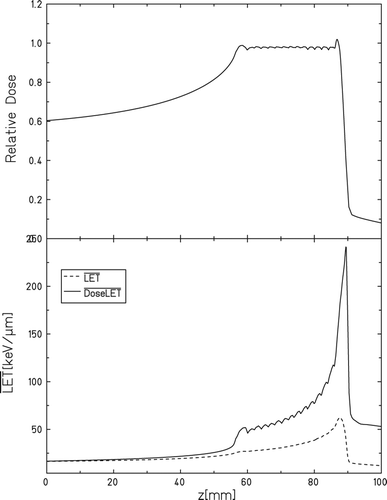
All carbon ion treatments were performed at the GSI synchrotron (SIS), using a 3 cm spread-out Bragg peak (SOBP), with the target placed 1.5 cm from the distal edge (). This position was selected to limit possible effects of uncertainty in achievable precision of positioning the target. At the distal end of the SOBP the LET gradient is the largest, which besides increasing the spread of LET across the tumor, also increases the sensitivity to the position and extent of the tumor. Therefore the tumor was placed closer to the center of the SOBP, which provides more robustness to our setup. The dose-average LET (LETD) at this position was calculated to be 65 keV/μm (57–78 keV/μm). The field sizes were 2 × 2.6 cm to guarantee full coverage of the tumor, as the position of the tumor on the leg could vary a bit from mouse to mouse. Additionally the field size was expanded laterally (to 2.5 × 2.6cm) to also cover a stack of Alanine pellets which were used as an extra dosimetry verification tool. Dose delivery at the GSI medical beamline is verified by multiple ionization chambers mounted in the beamline monitoring the scanning beam. Nonetheless, we did independent dosimetry verification by positioning stacks of Alanine pellets next to the tumors during radiation. The details on this and the necessary correction factors will be presented elsewhere.
X-ray irradiation of the reference group was performed at the Department of Experimental Clinical Oncology at Aarhus University Hospital. Irradiations were performed with a conventional therapeutic x-ray machine (240 kV; 10 mA; 2-mm Al filter; 1.1 mm Cu half-value layer; dose rate, 2.3 Gy/min). X-ray dose delivery for the reference group was monitored by use of an integrating chamber (Detector: TM31010, PTW-Freiburg).
The carbon ion irradiations and the x-ray irradiations were performed at the same time interval of the day to ensure no response differences due to the circadian rhythm of the mice.
Data analysis
For the dose response curves, all the lines through the data were determined by logit analysis. A Kaplan-Meier plot was used for fibrosis data. With the regrowth delay data, lines were fitted by eye, either using the mean from the dose group, or from individual animals.
Results
The dose response curves demonstrated a large difference on the effect of carbon ions versus photons. The TCD50 (dose producing tumor control in 50% of mice) values with 95% confidence interval were 29.7 (25.4–34.8) Gy for carbon ions and 43.9 (39.2–49.2) Gy for photons (). The corresponding RBE was 1.48 (1.28–1.72).
Figure 2. Tumor control. Effect of carbon ion irradiation on the radiation response of a C3H mouse mammary carcinoma. A: Tumor response was assessed by the percentage of mice showing local tumor control 90 days after treatment. The points represent results from an average of 5 or 6 mice per group and the errors (bars) show 95% confidence intervals on the TCD50 values, p < 0.0005. B: Tumor growth time of carbon ion irradiated tumors. For controls and 10–20 Gy (●) the results show means from an average of 5–9 mice per group. The curves are terminated when the first animals in the group had to be euthanized. Errors bars represents standard error (n = 5–9). For 30 Gy and 35 Gy (○) data from single tumors without local control is shown. Selected doses are shown for clarity. C: Tumor growth time of photon irradiated tumors. Results show means from an average of 5–9 mice per group. The curves are terminated when the first animals in the group had to be euthanized. Errors-bars represents standard error (n = 5–9).
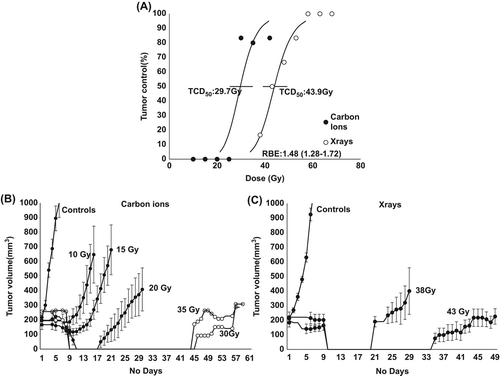
To illustrate the growth of the irradiated tumors, the tumor growth curves are shown from the same animals ( and ). The tumor growth was strongly dependent on the treatment dose, with a reduction in tumor size occurring around Day 7–11 after treatment for both radiation types.
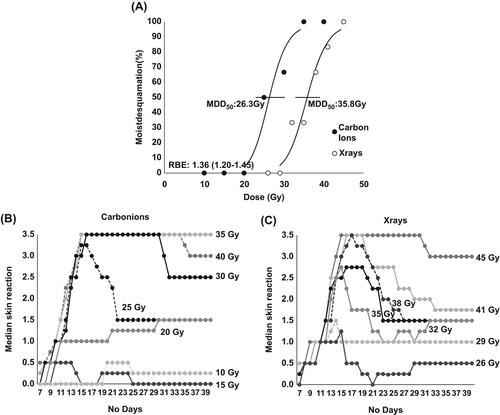
shows the dose-response curves for acute skin reaction for x-rays and carbon ions, with the percentage of animals in each treatment group showing moist desquamation (score 3.5) of the treated foot, at any time between Day 7 and 40. For acute skin damage the MDD50 (dose to produce moist desquamation in 50% of mice) values with 95% confidence interval were 26.3 (23.0–30.2) Gy for carbon ions and 35.8 (32.9–39.0) Gy for photons, resulting in a RBE of 1.36 (1.20–1.54). The development and decline of the early skin reactions following graded doses of radiation are shown in (carbon ions) and 3C (photons) as the median value within each dose group as a function of time. In regard to the time-dependency, induction of skin damage occurred around day 11 for both irradiation types with a maximum impact reached around Day 15–17 for dose groups reaching a median skin score value above 1.5.
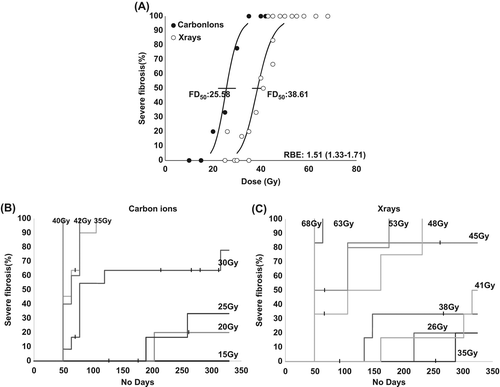
shows the late RIF at different doses for x-rays and carbon ions, with the percentage of animals in each treatment group with severe fibrosis of the treated foot, at any time between Day 49 and 322. For late tissue damage the FD50 (dose to produce severe fibrosis in 50% of mice) values with 95% confidence interval were 25.6 (22.3–29.4) Gy for carbon ions and 38.6 (36.4–40.9) Gy for photons, resulting in a RBE of 1.51 (1.33–1.71).
Discussion
RBE is used to express the increased effect of carbon ion radiation on biological systems, and depends on the radiation quality, commonly denoted as the dose-averaged LET of the beam of interest. We have in this study evaluated the effect of carbon ions on tumor control, acute skin damage and late radiation-induced fibrosis in CDF1 mice. , and shows the tumor control and the normal tissue reactions at different dose for x-rays and carbon ions. With high LET irradiation, the dose response curve shifted to the left for both tumor control and for acute skin damage. For all three endpoints, the time-dependency of the effect of x-ray irradiation and carbon ion radiation was similar. The three obtained RBE values where very similar.
The effect of high-LET radiation has been evaluated in a range of in vitro studies, demonstrating an increased RBE with an increasing LET, as reviewed in [Citation31,Citation32]. In cell experiments however, the cells are hit with well defined monoenergetic charged particles with the same LET. In vivo the cells are hit with a particle field of ions with a spectrum of energies and therefore various LET [Citation18]. Furthermore, cell experiments give good indications of the various effects, but are influenced by experimental constraints like plating efficiencies, which might influence the obtained data. In vivo a complex system of biological functions are interacting, which is impossible to mimic in vitro. In a clinical context, 1 fraction radiation scheme is not relevant, and obtained RBE values will most likely be higher in a fractionated radiation scheme [Citation22]. However, from a biological point of view, data obtained with 1 fraction is comparable between different studies, and makes it possible to distinguish between fractionation effects and other radiobiological effects.
When addressing the effectiveness of carbon ion therapy, the effect on the normal tissue is critical to the therapeutic dose that can be given. A significant increase of the RBE at high-LET would also enhance the unwanted side effects, which could compromise the gain achieved. Effects of radiation exposure on normal tissue consist of both early and late effects. Previous in vivo studies of the induction of damage in the normal tissue, includes an evaluation of the response of normal CNS tissue to high-LET irradiation in a series of rat spinal cord studies from Heidelberg [Citation33–35]. Amongst early effects, acute skin reactions, which are mostly reversible, are often seen. In previous studies by Ando et al. [Citation26,Citation27], evaluating the RBE of early skin reaction in mice irradiated with carbon ions, a RBE of around 2.2 at a LET of 42 keV/μm and around 2.5 at a LET of 77 keV/μm (for 1 fraction) was demonstrated. In the present study the RBE of early skin reaction, was found to be 1.36. The data is analyzed slightly differently in the two studies, with the dose response curves being calculated from the percentage of mice with moist desquamation, obtaining MDD50 values, in the present study, and from the average peak reaction in the study by Ando et al. However, analyzing our data in the same way as in the Ando study, results in a RBE comparable to the MDD50 RBE (data not shown). The difference in obtained RBE's may rather reflect difference in response between the mouse strains. Fibrosis is a common late damage effect of irradiation, which is irreversible and a dose limiting factor for healthy tissue in radiotherapy. An in vitro study evaluating the changes in fibrosis-related parameters after carbon ion irradiation did not find indications of higher RBE, when compared to other end-points [Citation36].
The effect on tumor response with carbon ion radiation has been the focus in a range of studies with tumor growth delay as endpoint [Citation26,Citation37–41]. Studies on the more clinically relevant endpoint of tumor control, has been the subject of a smaller number of studies [Citation20–22]. In the study by Peschke et al., a RBE of 2.3 was found in a radioresistant rat prostate tumor (Dunning subline R3327-AT1); mean dose-averaged LET of ∼75 keV/μm, with 1 fraction [Citation21]. Another study by Kummermehr et al., using a relatively slow growing mammary carcinoma (adenocarcinoma AT17) on C3H mice, found a RBE of 2.13. In the present study, the RBE for tumor control was 1.48. This lower observed RBE can be due to a range of factors, besides the differences in the LET. The RBE is influenced on the α/β ratio of the irradiated tissue [Citation42,Citation43], with the direct affect still being discussed. One hypothesis of the relationship between α/β ratio and RBE is that at high doses, fast growing tumors with high α/β ratios will display a lower RBE than slow growing tumors [Citation45]. This is supported by experimental data from in vitro experiments, pointing toward cells with lower α/β ratios to exhibit higher RBE values [Citation44]. The C3H mouse mammary carcinoma used in the present study is fast growing with a tumor doubling time of around 2.5 days [Citation46], with a high α/β ratio [Citation47]. A different issue affecting the RBE is the oxygen status of the irradiated tissue. With low-LET irradiation, low oxygen concentration in the irradiated tissue increase radioresistance [Citation48–50], which can be expressed as the oxygen enhancement ratio (OER). OER has been demonstrated to decrease with increasing LET [Citation51], with in vitro studies (10% survival) demonstrating an OER at 65 keV/μm at ∼2.2. Even though the very limited in vivo data though points towards a lower OER compared to the in vitro OER, it will still be influential and the degree of hypoxia in the included tumor models will affect the RBE determined. The hypoxic fraction in the C3H tumor is around 20%, with a ratio between normal or clamped conditions (complete hypoxic) of 1.19 [Citation52].
This study adds information to the variation in biological effectiveness in different tumor and normal tissue models. However, there is a need for a systematic range of in vivo studies on relevant models for both normal tissue complication and tumor response to uncover the effect of the biological variation.
Acknowledgements
The authors would like to thank Inger Marie Horsman and Dorthe Grand for excellent technical help and Marianne Verner Bjerre and Marianne Kristiansen for animal care. Financial support was received from Danish Cancer Society, Novo Nordisk, Andersons Cancer forskningsfond, Dansk Kræftforskningsfond, CIRRO – The Lundbeck Foundation Center for Interventional Research in Radiation Oncology and The Danish Council for Strategic Research.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Fokas E, Kraft G, An H, Engenhart-Cabillic R. Ion beam radiobiology and cancer: Time to update ourselves. Biochim Biophys Acta 2009;1796:216–29.
- Goodhead DT. Mechanisms for the biological effectiveness of high-LET radiations. J Radiat Res 1999;40(Suppl):1–13.
- Jäkel O. The relative biological effectiveness of proton and ion beams. Z Med Phys 2008;18:276–85.
- Elsässer T, Krämer M, Scholz M. Accuracy of the local effect model for the prediction of biologic effects of carbon ion beams in vitro and in vivo. Int J Radiat Oncol Biol Phys 2008;71:866–72.
- Mizoguchi N, Tsuji H, Toyama S, Kamada T, Tsujii H, Nakayama Y, et al. Carbon-ion radiotherapy for locally advanced primary or postoperative recurrent epithelial carcinoma of the lacrimal gland. Radiother Oncol 2015;114:373–7.
- Mizumoto M, Oshiro Y, Ayuzawa K, Miyamoto T, Okumura T, Fukushima T, et al. Preparation of pediatric patients for treatment with proton beam therapy. Radiother Oncol 2015; 114:245–8.
- Takagi M, Demizu Y, Hashimoto N, Mima M, Terashima K, Fujii O, et al. Treatment outcomes of particle radiotherapy using protons or carbon ions as a single-modality therapy for adenoid cystic carcinoma of the head and neck. Radiother Oncol 2014;113:364–70.
- Koto M, Hasegawa A, Takagi R, Sasahara G, Ikawa H, Mizoe J-E, et al. Feasibility of carbon ion radiotherapy for locally advanced sinonasal adenocarcinoma. Radiother Oncol 2014;113:60–5.
- Ladra MM, Edgington SK, Mahajan A, Grosshans D, Szymonifka J, Khan F, et al. A dosimetric comparison of proton and intensity modulated radiation therapy in pediatric rhabdomyosarcoma patients enrolled on a prospective phase II proton study. Radiother Oncol 2014;113:77–83.
- Combs SE, Kessel K, Habermehl D, Haberer T, Jäkel O, Debus J. Proton and carbon ion radiotherapy for primary brain tumors and tumors of the skull base. Acta Oncol 2013;52:1504–9.
- Combs SE, Debus J. Treatment with heavy charged particles: Systematic review of clinical data and current clinical (comparative) trials. Acta Oncol 2013;52:1272–86.
- Yock TI, Constine LS, Mahajan A. Protons, the brainstem, and toxicity: Ingredients for an emerging dialectic. Acta Oncol 2014;53:1279–82.
- Indelicato DJ, Flampouri S, Rotondo RL, Bradley JA, Morris CG, Aldana PR, et al. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol 2014;53:1298–304.
- Cuaron JJ, Harris AA, Chon B, Tsai H, Larson G, Hartsell WF, et al. Anterior-oriented proton beams for prostate cancer: A multi-institutional experience. Acta Oncol 2015;54:868–74.
- Wilkens JJ, Oelfke U. Direct comparison of biologically optimized spread-out Bragg peaks for protons and carbon ions. Int J Radiat Oncol Biol Phys 2008;70:262–6.
- Grün R, Friedrich T, Krämer M, Zink K, Durante M, Engenhart-Cabillic R, et al. Assessment of potential advantages of relevant ions for particle therapy: A model based study. Med Phys 2015;42:1037–47.
- Schlaff CD, Krauze A, Belard A, O’Connell JJ, Camphausen KA. Bringing the heavy: Carbon ion therapy in the radiobiological and clinical context. Radiat Oncol 2014;9:88.
- Skarsgard LD. Radiobiology with heavy charged particles: A historical review. Phys Med 1998;14(Suppl 1):1–19.
- Durante M. New challenges in high-energy particle radiobiology. Br J Radiol 2014;87:20130626.
- Kummermehr J, Palme B, Galwas K, Sanchez-Brandelik R, Scholz M. Response of a mammary carcinoma in vivo to single dose irradiation with 2cm SOBP carbon ions. GSI Sci Rep 2002.
- Peschke P, Karger CP, Scholz M, Debus J, Huber PE. Relative biological effectiveness of carbon ions for local tumor control of a radioresistant prostate carcinoma in the rat. Int J Radiat Oncol Biol Phys 2011;79:239–46.
- Karger CP, Peschke P, Scholz M, Huber PE, Debus J. Relative biological effectiveness of carbon ions in a rat prostate carcinoma in vivo: Comparison of 1, 2, and 6 fractions. Int J Radiat Oncol Biol Phys 2013;86:450–5.
- Overgaard J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int J Radiat Oncol Biol Phys 1980;6:1507–17.
- Horsman MR, Chaplin DJ, Overgaard J. Combination of nicotinamide and hyperthermia to eliminate radioresistant chronically and acutely hypoxic tumor cells. Cancer Res 1990;50:7430–6.
- Horsman MR, Siemann DW, Chaplin DJ, Overgaard J. Nicotinamide as a radiosensitizer in tumours and normal tissues: The importance of drug dose and timing. Radiother Oncol 1997;45:167–74.
- Ando K, Koike S, Uzawa A, Takai N, Fukawa T, Furusawa Y, et al. Biological gain of carbon-ion radiotherapy for the early response of tumor growth delay and against early response of skin reaction in mice. J Radiat Res 2005;46:51–7.
- Ando K, Koike S, Nojima K, Chen YJ, Ohira C, Ando S, et al. Mouse skin reactions following fractionated irradiation with carbon ions. Int J Radiat Biol 1998;74:129–38.
- Von der Maase H. Effect of cancer chemotherapeutic drugs on the radiation-induced skin reactions in mouse feet. Br J Radiol 1984;57:697–707.
- Stone HB. Leg contracture in mice: An assay of normal tissue response. Int J Radiat Oncol Biol Phys 1984;10:1053–61.
- Nawroth I, Alsner J, Behlke MA, Besenbacher F, Overgaard J, Howard KA, et al. Intraperitoneal administration of chitosan/DsiRNA nanoparticles targeting TNFα prevents radiation-induced fibrosis. Radiother Oncol 2010;97:143–8.
- Friedrich T, Scholz U, Elsässer T, Durante M, Scholz M. Systematic analysis of RBE and related quantities using a database of cell survival experiments with ion beam irradiation. J Radiat Res 2013;54:494–514.
- Sørensen BS, Overgaard J, Bassler N. In vitro RBE-LET dependence for multiple particle types. Acta Oncol 2011; 50:757–62.
- Karger CP, Peschke P, Sanchez-Brandelik R, Scholz M, Debus J. Radiation tolerance of the rat spinal cord after 6 and 18 fractions of photons and carbon ions: Experimental results and clinical implications. Int J Radiat Oncol Biol Phys 2006;66:1488–97.
- Debus J, Scholz M, Haberer T, Peschke P, Jäkel O, Karger CP, et al. Radiation tolerance of the rat spinal cord after single and split doses of photons and carbon ions. Radiat Res 2003; 160:536–42.
- Saager M, Glowa C, Peschke P, Brons S, Scholz M, Huber PE, et al. Carbon ion irradiation of the rat spinal cord: Dependence of the relative biological effectiveness on linear energy transfer. Int J Radiat Oncol Biol Phys 2014;90:63–70.
- Fournier C, Scholz M, Weyrather WK, Rodemann HP, Kraft G. Changes of fibrosis-related parameters after high- and low-LET irradiation of fibroblasts. Int J Radiat Biol 2001;77:713–22.
- Takahashi A, Yano T, Matsumoto H, Wang X, Ohnishi K, Tamamoto T, et al. Effects of accelerated carbon-ions on growth inhibition of transplantable human esophageal cancer in nude mice. Cancer Lett 1998;122:181–6.
- Kitabayashi H, Shimada H, Yamada S, Yasuda S, Kamata T, Ando K, et al. Synergistic growth suppression induced in esophageal squamous cell carcinoma cells by combined treatment with docetaxel and heavy carbon-ion beam irradiation. Oncol Rep 2006;15:913–8.
- Asakawa I, Yoshimura H, Takahashi A, Ohnishi K, Nakagawa H, Ota I, et al. Radiation-induced growth inhibition in transplanted human tongue carcinomas with different p53 gene status. Anticancer Res 22:2037–43.
- Subtil FSB, Wilhelm J, Bill V, Westholt N, Rudolph S, Fischer J, et al. Carbon ion radiotherapy of human lung cancer attenuates HIF-1 signaling and acts with considerably enhanced therapeutic efficiency. FASEB J 2014;28: 1412–21.
- Koike S, Ando K, Oohira C, Fukawa T, Lee R, Takai N, et al. Relative biological effectiveness of 290 MeV/u carbon ions for the growth delay of a radioresistant murine fibrosarcoma. J Radiat Res 2002;43:247–55.
- Paganetti H, Gerweck LE, Goitein M. The general relation between tissue response to x-radiation (alpha/beta-values) and the relative biological effectiveness (RBE) of protons: Prediction by the Katz track-structure model. Int J Radiat Biol 2000;76:985–98.
- Hawkins RB. A microdosimetric-kinetic model for the effect of non-Poisson distribution of lethal lesions on the variation of RBE with LET. Radiat Res 2003;160:61–9.
- Gerweck LE, Kozin S V. Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol 1999; 50:135–42.
- Jones B, Wilson P, Nagano A, Fenwick J, McKenna G. Dilemmas concerning dose distribution and the influence of relative biological effect in proton beam therapy of medulloblastoma. Br J Radiol 2012;85:e912–8.
- Horsman MR, Murata R. Vascular targeting effects of ZD6126 in a C3H mouse mammary carcinoma and the enhancement of radiation response. Int J Radiat Oncol Biol Phys 2003;57:1047–55.
- Williams M V, Denekamp J, Fowler JF. A review of alpha/beta ratios for experimental tumors: Implications for clinical studies of altered fractionation. Int J Radiat Oncol Biol Phys 1985;11:87–96.
- Höckel M, Schlenger K, Mitze M, Schäffer U, Vaupel P. Hypoxia and radiation response in human tumors. Semin Radiat Oncol 1996;6:3–9.
- Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol 1996;41:31–9.
- Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953; 26:638–48.
- Wenzl T, Wilkens JJ. Modelling of the oxygen enhancement ratio for ion beam radiation therapy. Phys Med Biol 2011;56:3251–68.
- Horsman MR, Grau C, Overgaard J. Reoxygenation in a C3H mouse mammary carcinoma. The importance of chronic rather than acute hypoxia. Acta Oncol 1995;34:325–8.

