ABSTRACT
Background. To determine by treatment plan comparison differences in toxicity risk reduction for patients with head and neck squamous cell carcinoma (HNSCC) from proton therapy either used for complete treatment or sequential boost treatment only.
Materials and methods. For 45 HNSCC patients, intensity-modulated photon (IMXT) and proton (IMPT) treatment plans were created including a dose escalation via simultaneous integrated boost with a one-step adaptation strategy after 25 fractions for sequential boost treatment. Dose accumulation was performed for pure IMXT treatment, pure IMPT treatment and for a mixed modality treatment with IMXT for the elective target followed by a sequential boost with IMPT. Treatment plan evaluation was based on modern normal tissue complication probability (NTCP) models for mucositis, xerostomia, aspiration, dysphagia, larynx edema and trismus. Individual NTCP differences between IMXT and IMPT (∆NTCPIMXT-IMPT) as well as between IMXT and the mixed modality treatment (∆NTCPIMXT-Mix) were calculated.
Results. Target coverage was similar in all three scenarios. NTCP values could be reduced in all patients using IMPT treatment. However, ∆NTCPIMXT-Mix values were a factor 2–10 smaller than ∆NTCPIMXT-IMPT. Assuming a threshold of ≥ 10% NTCP reduction in xerostomia or dysphagia risk as criterion for patient assignment to IMPT, less than 15% of the patients would be selected for a proton boost, while about 50% would be assigned to pure IMPT treatment. For mucositis and trismus, ∆NTCP ≥ 10% occurred in six and four patients, respectively, with pure IMPT treatment, while no such difference was identified with the proton boost.
Conclusions. The use of IMPT generally reduces the expected toxicity risk while maintaining good tumor coverage in the examined HNSCC patients. A mixed modality treatment using IMPT solely for a sequential boost reduces the risk by 10% only in rare cases. In contrast, pure IMPT treatment may be reasonable for about half of the examined patient cohort considering the toxicities xerostomia and dysphagia, if a feasible strategy for patient anatomy changes is implemented.
Proton therapy (PT) of patients with head and neck squamous cell carcinoma (HNSCC) allows for the reduction of dose to the surrounding organs at risk (OAR) compared to photon therapy (XT) [Citation1,Citation2]. This may translate to reduced incidence of toxicities after radiotherapy treatment. However, the use of PT is largely restricted by the availability of PT centers. With respect to these limited PT resources, a larger number of patients could be treated with PT, if only the sequential boost is given with protons. Such a treatment approach would be reasonable, if there is an expected advantage compared to conventional XT treatment.
Another potential advantage of using PT for the sequential boost only is the reduced risk of degraded dose distributions in PT caused by anatomical changes of the patient during the whole treatment course of several weeks due to the reduced treatment time spent for PT.
The use of ion therapy for sequential boost treatment was investigated for malignant salivary gland tumors by several groups showing the feasibility in terms of acceptable toxicity and tumor control [Citation3,Citation4]. A comparison to toxicities in standard radiotherapy was not performed. Thus, the benefit of PT for a partial treatment compared to conventional XT and complete PT was not evaluated and yet remains unclear.
Recently, Jakobi et al. [Citation5] presented a treatment planning study comparing intensity-modulated XT (IMXT) and intensity-modulated PT (IMPT) for locally advanced HNSCC patients aiming at the identification of patients with larger benefit from PT using normal tissue complication probability (NTCP) models. Here, we present a detailed treatment plan analysis for the same cohort of 45 patients comparing toxicity risk in terms of NTCP when using IMXT followed by a sequential IMPT boost. These results are compared to a pure IMXT and a pure IMPT treatment for evaluation of the potential benefit for the patient.
Material and methods
Patient selection, treatment schedule, volume definition, treatment planning
Imaging data included computed tomography (CT) and FDG positron emission tomography (PET) for 45 patients with HNSCC. All patients gave written consent for the use of their data. The study was approved by the local ethics committee.
According to a planned multi-centric clinical trial, a treatment schedule was defined, which consists of two main treatment series planned on two different CT datasets: First, the treatment series of 25 fractions for the elective target volume on a baseline CT with 2 Gy per fraction plus a simultaneous integrated boost (SIB) to the gross tumor volume (GTV), starting after 10 fractions with 2.3 Gy. Second, a sequential boost of 11 fractions on a follow-up FDG-PET/CT scan taken after 20 fractions was planned with 2 Gy to the GTV and suspect lymph nodes plus a SIB to FDG avid volumes inside the GTV. An elective clinical target volume (CTVelec) was created for the first treatment series including lymphatic pathways and a CTVboost as expansion of the GTV for subclinical spread. The CTVs were expanded by 5 mm in cranio-caudal direction and 4 mm in plane, retaining a 3 mm distance to the external contour for creation of the planning target volume (PTV). OARs were only delineated outside the GTV and when a substantial portion was not infiltrated (physician's decision). Delineated OAR were spinal cord (45 patients), brain stem (45 patients), parotid glands (45 patients), brachial plexus (45 patients), mucosa (45 patients), swallowing muscles (45 patients), larynx (37 patients), esophagus (45 patients), mandible (44 patients), temporomandibular joints (44 patients), submandibular (44 patients contralateral, 43 ipsilateral) and sublingual glands (41 patients contralateral, 36 ipsilateral).
IMPT treatment planning was based on a standard set of three coplanar angles (−40°, 40°, 180°), which were adapted to the individual patient anatomy (e.g. for one-sided sequential boost volumes). A constant correction factor of 1.1 was used for the higher relative biological effectiveness of protons compared to photons, such that all values given in Gy actually mean GyE for PT. Step and shoot IMXT planning was done with seven coplanar, equally distributed beam angles. The beam number was reduced to five for the sequential boost in case of a one-sided sequential boost volume. Plan optimization had to fulfill V95% > 95% for the planning target volumes, while minimizing V107% and aiming for a homogeneous dose distribution with the mean dose close to the prescribed dose. Target goals were compromised if necessary to achieve dose constraints of the spinal cord (Dmax < 45 Gy), brain stem (Dmax < 54 Gy) and brachial plexus (Dmax < 72 Gy). For other OAR like parotids, larynx, oral cavity and mandible the dose was minimized without compromising tumor coverage. Dose constraints for the OAR were scaled for the two series according to the planned total dose. The constraints had to be fulfilled independently in the respective series plans without considering possible dose reduction in the sequential series. This allowed for the creation of the mixed modality treatment plans (IMXT followed by IMPT boost) by summation of the IMXT plan for the elective volume and the IMPT plan for the sequential boost without further optimization. Treatment planning for the presented treatment planning study was independent of the actual clinical treatment of the patients. A more detailed description of the patient characteristics, treatment schedule, target definition and treatment planning is presented in Jakobi et al. [Citation5].
Evaluation of treatment plans
Dose accumulation of the treatment plan series was performed on the baseline CT using a deformable registration based on the respective CTs using a diffeomorphic demon algorithm implemented in the software package 3DSlicer [Citation6]. The image registration was validated based on a qualitative assessment with vector field visualization and a quantitative assessment using contour comparisons and dose value comparisons [Citation7]. The cumulative treatment dose was used for OAR evaluation, while target parameters were evaluated separately for IMXT and IMPT on the CT dataset which contained the respective volume (elective PTV on baseline CT, sequential boost volume on in-treatment CT). Dose conformity for the respective target volumes was evaluated with the conformity number (CN) [Citation8]. Perfect conformity is represented by CN = 1 and lower values describe decreasing conformity.
Biological evaluation was done for several clinical endpoints based on modern NTCP models with both, underlying patient characteristics and treatment procedures similar to the presented study: Incidence of acute oral mucositis (grade ≥ 3) [Citation9], aspiration assessed by videofluoroscopy [Citation10], xerostomia 12 months after therapy [Citation11], subjective and objective swallowing dysfunction [Citation12], late larynx edema (grade ≥ 2) [Citation13] and trismus assessed as jaw-opening < 35 mm [Citation14], cf. Jakobi et al. [Citation5] for details.
Individual matched-pair NTCP differences between a patient's IMXT and IMPT treatment plan (∆NTCPIMXT-IMPT) as well as between IMXT and mixed modality treatment plans (∆NTCPIMXT-Mix) were determined. These differences were tested for statistical significance using two-sided paired t-tests with a significance level of 0.05. A benefit for IMPT or the mixed modality treatment was defined as an individual ∆NTCPIMXT-IMPT or ∆NTCPIMXT-Mix ≥ 10%, similar to the model-based approach for patient identification proposed by Langendijk et al. [Citation15].
Results
Physical dose characteristics
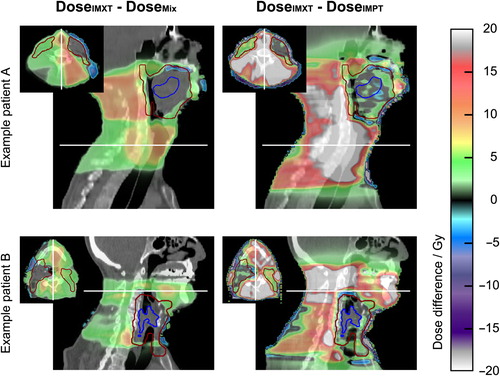
The IMXT and IMPT treatment plans were of similar quality with respect to target dose coverage parameters evaluated on both CT datasets. The median difference of individual V95%,IMXT − V95%,IMPT was less than 1%. The median difference of individual V107%,IMXT − V107%,IMPT in the PTV excluding the SIB volume expanded by 5 mm was 0%. The prioritized dose constraints for spinal cord, brain stem and brachial plexus were fulfilled with both IMXT and IMPT. The integral dose outside the target volume (total amount of absorbed energy) with IMXT was about twice the dose with IMPT for all patients, highlighting the generally reduced normal tissue dose achievable with IMPT. Similarly, the CN was lower with on average 0.75 ± 0.04 for IMXT compared to the IMPT plans with CN = 0.83 ± 0.03, reflecting the less conformal dose of the IMXT treatments. Dose differences between IMXT and IMPT and between IMXT and the mixed modality treatment are illustrated in for two exemplary patients.
Figure 1. Dose differences overlaid on a sagittal and axial CT slice (slice positions are indicated by white lines) between pure IMXT plan and mixed modality treatment plan (left column) as well as between pure IMXT treatment and pure IMPT treatment (right column) for two exemplary patients with primary tumor site in the oropharynx (upper row) and the hypopharynx (lower row). The elective target volume is depicted in dark red and the gross tumor volume (GTV) in blue. Dose differences are small in the sequential boost volume consisting of GTV and involved lymph nodes (not depicted), increasing in the part of the elective volume in which no sequential boost is applied (right neck in patient A, left neck in patient B) and largest in the normal tissue surrounding the elective volume.
NTCP evaluation
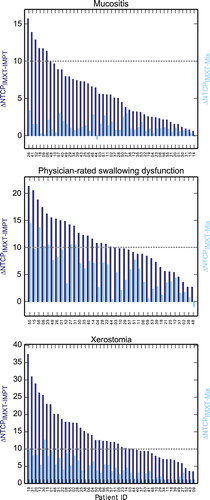
With only few exceptions, NTCP values were reduced in all patients with pure IMPT treatment and IMPT boost treatment compared to IMXT treatment. The exceptions occurred in single patients for the toxicities aspiration (two patients with IMPT boost), swallowing dysfunction (two patients with IMPT boost), larynx edema (one patient with IMPT boost), mucositis (two patients with IMPT boost) and trismus (one patient with pure IMPT), cf. patients with negative values in and Supplementary Figure 1 (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1071920). NTCPMix values were significantly higher than NTCPIMPT values, but still significantly lower than NTCPIMXT (p < 0.001 for all NTCP models). The magnitude of the differences was patient and toxicity dependent. This is shown exemplarily in for the three toxicities mucositis, physician-rated swallowing dysfunction and xerostomia. For the other four toxicities, similar images can be found in the Supplementary Figure 1 available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1071920.
Figure 2. Differences in NTCP between pure IMXT and pure IMPT (∆NTCPIMXT-IMPT, dark blue) and between pure IMXT and mixed modality treatment (∆NTCPIMXT-Mix, light blue) for individual patients sorted according to the ∆NTCPIMXT-IMPT values for three different toxicities. The gray dotted line indicates 10% difference used as possible selection criterion in the model-based approach.
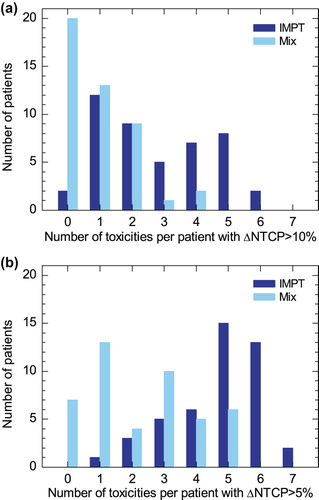
Using a threshold of ∆NTCPIMXT-Mix ≥ 10% as treatment selection criteria for PT, the sequential proton boost would lead to a selection of only 0, 0, 2, 25, 6, 2 and 7 patients for oral mucositis, trismus, xerostomia, aspiration, physician-rated swallowing dysfunction, patient-rated problems with swallowing solid food and larynx edema, respectively. In contrast, ∆NTCPIMXT-IMPT ≥ 10% was achieved for these toxicities in additional 6, 4, 26, 10, 13, 12 and 12 cases, respectively, indicating the higher probability of toxicity reduction with pure IMPT. The number of patients with ∆NTCP ≥ 10%, 5–10% and ≤ 5% is given in for pure IMPT and the mixed modality treatment.
Table I. Number of patients with ∆NTCP in the specified ranges.
In addition to the toxicity-wise evaluation, an individual patient assessment was performed by determining the number of the seven examined toxicities with ∆NTCP ≥ 10% per patient, cf. . The large difference in toxicity risk reduction between the two proton techniques, pure IMPT on the one hand and the proton boost on the other hand, was reflected in this evaluation. ∆NTCP ≥ 10% for maximum one of the toxicities occurred in 33 of 45 patients with the proton boost, indicating the small effect of this technique in most of the patients. Such a small number of toxicities with a relevant NTCP reduction was only the case for 14 patients when using pure IMPT. In contrast, with pure IMPT a NTCP reduction ≥ 10% for at least three toxicities was possible for 22 of 45 patients, but only three patients showed this desirable difference using the mixed modality treatment. Applying an alternative selection criterion of ∆NTCP > 5% revealed the same tendency for the different scenarios, cf. .
Discussion
The presented treatment planning study was performed to estimate the potential benefit of a sequential proton boost in terms of toxicity risk reduction for patients with advanced HNSCC. With the presented treatment schedule, three patients could be treated with a sequential boost treatment in the same time slots at the treatment machine, instead of only one patient with pure IMPT.
The use of IMPT reduced the probability of toxicity incidence in both scenarios compared to IMXT. However, the effect was small using IMPT only for a sequential boost treatment following IMXT of the elective volume compared to the complete treatment with IMPT. For all NTCP models, except for aspiration, the fraction of patients eligible for referral to a sequential proton boost according to a threshold of ∆NTCPIMXT-Mix ≥ 10 % was between 0% and 15%, while for a pure proton treatment 10% to 60% of the patients were eligible. These numbers might be influenced by the distribution of the tumor location in the investigated patient cohort and could deviate for a different patient cohort composition. However, the general tendency of the reduced benefit using IMPT for sequential boost appears to be independent of the patient cohort composition as almost all patients showed a higher NTCP reduction with pure IMPT. The number of toxicities with reduced risk for the individual patient was higher with pure IMPT, where for almost half of the patients at least three NTCP models showed a reduction of more than 10%, compared to three of 45 patients with PT boost only. The small NTCP benefit for the sequential boost treatment with PT results from the large elective volume, which is treated with photons in both, pure IMXT and mixed modality treatment, resulting in a high integral dose outside the target volume. The sequential boost treatment adds only about a third of the prescribed total dose and is confined to a smaller anatomical region, as it is focused on the GTV. As a consequence, OARs which are distant to the GTV will encounter only slightly increased dose with an additional sequential boost treatment. Thereby, the effect of the more conformal proton dose distribution is reduced when used only for boost treatment. For this reason, ∆NTCPIMXT-Mix ≥ 10% occurs for patients whose tumor is localized close to the OAR relevant for the specific side effect. However, even for those patients, the use of pure IMPT would reduce the toxicity risk by a higher value. The possibility to offer PT to more patients when using the technique only for the sequential boost may not outweigh the reduced benefit in terms of NTCP reduction.
A limitation of the presented results is the use of NTCP models that were not specifically validated for the presented patient cohort and treatment technique. As the model-based approach [Citation15] depends on the validity of the underlying NTCP models, this may induce uncertainties to the results. Thus, the treatment may lead to different toxicity rates than estimated here. However, the model choice was limited to models generated from similar patient cohorts and similar photon treatment techniques. The obtained NTCP values are in the expected range and thus using the selected models seems reasonable for the presented analysis. Furthermore, while other models may lead to different absolute NTCP values, the observed overall trend in the relative differences might be robust.
Another limitation is the use of two image datasets for treatment planning and dose evaluation. On the one hand, the use of a second image dataset acquired during treatment for treatment plan adaptation is a reasonable approach for PT which is highly sensible to anatomical changes of the patient. However, the use of two image datasets still represents only two snapshots of the patient. Thus, the evaluated dose distribution is a nominal one and may not be a good representation of the “dose of the day” in case of large anatomical changes, which can be subject to higher dose degradations [Citation16]. On the other hand, the use of more than one image dataset required a deformable image registration for dose accumulation. Although, the registration had been validated and was deemed of good quality, additional uncertainties in the dose distribution are introduced by the use of deformed dose cubes. However, these uncertainties are similarly present in the IMPT and IMXT treatment plans.
In conclusion, IMPT used for complete treatment or as sequential boost option reduced the expected toxicity risk compared to IMXT while maintaining good tumor coverage in the examined HNSCC patients. The mixed modality treatment using IMPT solely for sequential boosting offers a desired minimum risk reduction of ≥ 10% only in rare cases. In contrast, pure IMPT treatment may be beneficial in terms of expected toxicity risk reduction for the main toxicities xerostomia and dysphagia for about half of the examined patient cohort. The implementation of pure PT for HNSCC patients requires a feasible protocol for handling anatomical changes of the patient throughout the treatment course. The availability of PT to a larger number of patients and the minor toxicity reduction for a sequential proton boost treatment of HNSCC patients need to be balanced in clinical practice. Based on the presented analysis pure PT would be preferential in the clinical setting.
Supplementary material available online
Supplementary Figure 1 available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2015.1071920
ionc_a_1071920_sm9732.zip
Download Zip (165 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- van de Water TA, Lomax AJ, Bijl HP, de Jong ME, Schilstra C, Hug EB, et al. Potential benefits of scanned intensity-modulated proton therapy versus advanced photon therapy with regard to sparing of the salivary glands in oropharyngeal cancer. Int J Radiat Oncol Biol Phys 2011; 79:1216–24.
- van der Laan HP, van de Water TA, van Herpt HE, Christianen MEMC, Bijl HP, Korevaar EW, et al. The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: A planning comparative study. Acta Oncol 2013; 52:561–9.
- Schulz-Ertner D, Nikoghosyan A, Didinger B, Münter M, Jäkel O, Karger CP, et al. Therapy strategies for locally advanced adenoid cystic carcinomas using modern radiation therapy techniques. Cancer 2005;104:338–44.
- Jensen AD, Nikoghosyan AV, Lossner K, Herfarth KK, Debus J, Münter MW. IMRT and carbon ion boost for malignant salivary gland tumors: Interim analysis of the cosmic trial. BMC Cancer 2012;12:163.
- Jakobi A, Bandurska-Luque A, Stützer K, Haase R, Löck S, Wack LJ, et al. Identification of patient benefit from proton therapy for advanced head and neck cancer patients based on individual and subgroup NTCP analysis. Int J Radiat Oncol Biol Phys 2015;92:1165–74.
- Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3Dd slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 2012;30:1323–41.
- Stützer K, Jakobi A, Haase R, Bandurska-Luque A, Löck S, Krause M, et al. Characterization of deformation vector fields for the registration of dose distributions in adaptive treatment planning and sequential boost protocols. In: Klöck S, editor. Abstractbook – Joint Conference of the SSRMP, DGMP, ÖGMP – Dreiländertagung der Medizinischen Physik. 2014:140–1. Available from: http://domains.conventus.de/fileadmin/media/2014/dgmp/MedPhys2014_Abstractbook.pdf. [cited 2015 May 04]
- Van't Riet A, Mak AC, Moerland MA, Elders LH, van der Zee W. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys 1997;37:731–6.
- Bhide SA, Gulliford S, Schick U, Miah A, Zaidi S, Newbold K, et al. Dose-response analysis of acute oral mucositis and pharyngeal dysphagia in patients receiving induction chemotherapy followed by concomitant chemo-IMRT for head and neck cancer. Radiother Oncol 2012; 103:88–91.
- Eisbruch A, Kim HM, Feng FY, Lyden TH, Hacer MJ, Denk M, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: Swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys 2011;81:e93–9.
- Houweling AC, Philippens MEP, Dijkema T, Roesink JM, Terhaard CH, Schilstra C, et al. A comparison of dose- response models for the parotid gland in a large group of head-and-neck cancer patients. Int J Radiat Oncol Biol Phys 2010;76:1259–65.
- Christianen MEMC, Schilstra C, Beetz I, Mujis CT, Chouvalova O, Burlage FR, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: Results of a prospective observational study. Radiother Oncol 2012;105:107–14.
- Rancati T, Fiorino C, Sanguineti G. NTCP modeling of subacute/late laryngeal edema scored by fiberoptic examination. Int J Radiat Oncol Biol Phys 2009;75:915–23.
- Lindblom U, Gärskog O, Kjellén E, Laurell G, Levring Jäghagen E, Wahlberg P, et al. Radiation-induced trismus in the ARTSCAN head and neck trial. Acta Oncol 2014;53:620–7.
- Langendijk JA, Lambin P, De Ruysscher D, Widder J, Bos M, Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model-based approach. Radiother Oncol 2013;107:267–73.
- Müller BS, Duma MS, Kampfer S, Nill S, Oelfke U, Geinitz H, et al. Impact of interfractional changes in head and neck cancer patients on the delivered dose in intensity modulated radiotherapy with protons and photons. Phys Med 2015;31:266–72.