Abstract
Background: Voluntary moderate deep inspiration breath-hold (vmDIBH) is widely used for left sided breast cancer patients. The purpose of this study was to investigate the usefulness of vmDIBH in local and locoregional radiation therapy (RT) of right-sided breast cancer.
Materials and Methods: For fourteen right-sided breast cancer patients, 3D-conformal (3D-CRT) RT plans (i.e., forward IMRT) were calculated on free-breathing (FB) 3D-CRT(FB) and vmDIBHCT-scans, for local- as well as locoregional breast treatment, with and without internal mammary nodes (IMN). Dose volume parameters were compared.
Results: For local breast treatment, no relevant reduction in mean lung dose (MLD) was found. For locoregional breast treatment without IMN, the average MLD reduced from 6.5 to 5.4 Gy (p < 0.005) for the total lung and from 11.2 to 9.7 Gy (p < 0.005) for the ipsilateral lung. For locoregional breast treatment with IMN, the average MLD reduced from 10.8 to 9.1 Gy (p < 0.005) for the total lung and from 18.7 to 16.2 Gy (p < 0.005) for the ipsilateral lung, whilea small reduction in mean heart dose of 0.4 Gy (p = 0.07) was also found.
Conclusions: Breathing adapted radiation therapy in left-sided breast cancer patients is becoming widely introduced. As a result of the slight reduction in lung dose found for locoregional right-sided breast cancer treatment in this study, a slightly lower risk of pneumonitis and secondary lung cancer (in ever smoking patients) can be expected.In addition, for some patients the heart dose will also be reduced by more than 0.5 up to 2.6 Gy. We therefore suggest to also apply breath-hold for locoregional irradiation of right-sided breast cancer patients.
Introduction
Radiation therapy (RT) has demonstrated clear clinical benefits for all patients treated with breast conserving therapy (BCT) and for patients after radical mastectomy with risk factors [Citation1]. In addition, locoregional RT is reported to improve disease free and overall survival for patients with involved axillary or internal mammary lymph nodes [Citation2].
However, it is impossible to totally avoid incidental irradiation of the lungs and heart with conventional RT techniques, for local breast irradiation and especially when the axillary or internal mammary lymph nodes (IMN) are included in the target volume [Citation3]. There are concerns about the acute and late pulmonary and cardiovascular toxicity, especially for patients with a medical history of cardiovascular and/or pulmonary disease [Citation4,Citation5].
A common practice nowadays to reduce the cardiac dose and therefore the risk of cardiac injury is to apply breath-hold techniques for locally and locoregionally irradiated left-sided breast cancer patients [Citation8–11]. Different methods are used to monitor and control respiratory motion (e.g. Real-time Position Management system (RPM) system [Citation8], active breathing control [Citation9], and voluntary moderate deep inspiration breath-hold (vmDIBH) [Citation11] and were found to result in comparable dosimetric benefits.
Besides cardiac dose reduction as a result of breathing adapted RT many studies mention the reduction in lung dose and as a result the possible reduction in lung toxicity, mainly radiation pneumonitis [Citation7,Citation12]. Therefore, breathing adapted RT might also be beneficial for right-sided breast cancer patients, which is only briefly mentioned and studied in a few papers. As part of the studied patient population, two research groups noticed a difference in the lung dose in FB and breath-hold for right-sided breast cancer patients [Citation6,Citation7,Citation12]. As one of the first investigators, Remouchamps et al. [Citation7] presented a treatment planning comparison for 9 left-sided and 6 right-sided breast cancer patients. The pooled results for these patients showed that the mean percentage of both lungs receiving more than 20 Gy was reduced from 20.4% in IMRT-FB to 15.2% in IMRT-mDIBH. Korreman et al. [Citation6] showed pooled results for 9 left-sided and 8 right-sided breast cancer patients treated locoregionally including the IMN: the median ipsilateral relative lung volume receiving > 50% of the prescribed CTV dose was reduced from 45.6% (23.5–66.2%) in FB to 23.2% (15.2–42.8%) in DIBH. In their 2006 paper the authors show from the pooled data of the previous study that the pneumonitis probability of 28.1% (range, 0.7–95.6%) for FB could be reduced to 4.3% (range, 0.1–59%) for DIBH [Citation12].
The purpose of this study was to investigate the usefulness of breathing adapted RT for right-sided breast cancer patients, both when treated locally and locoregionally, with or without IMN. The reduction in irradiated lung dose/volume and possible effect on pulmonary risks were studied.
Methods and Materials
In this study, fourteen patients (mean age = 57 years, range 34 – 80 years, 3 patients < 50 years) with a CT-scan in breath-hold (vmDIBH) and in free breathing (FB) were included. The vmDIBH scans were acquired with breathing instructions and were verified using breathing signals from the Real-Time Position Management (RPM) respiratory gating system (Varian Medical Systems). The CT slice thickness was 2.5mm. For each patient, three clinical target volumes (CTVs) were delineated (and checked on both scans) by one of the radiation oncologists (DC) based on the ESTRO guidelines [Citation3]: 1) local right sided breast, 2) locoregional right-sided breast, including axilla levels I and II, and axilla levels III-IV (formally known as periclavicular), and 3) locoregional right-sided breast including axilla levels I to IV and the internal mammary nodes. The planning target volumes (PTVs) were constructed by adding a 5mm margin to the CTV, except for superficial areas where the PTV was cropped up to 5 mm from the surface of the skin. The 5mm margin was based on the determined residual setup error after on-line setup for a group of 20 patients treated in vmDIBH (group mean = 1.8mm, systematic error = 1.4mm, random error = 1.6mm [Citation11]. The following normal structures were contoured; patient body, contralateral breast, heart, left lung, right lung, and combined lungs. The prescribed dose (Dpresc) was 42.56Gy in 16 fractions.
For each patient and each PTV, two treatment plans were generated in the Eclipse TPS (Acuros version 10.0): 1) a 3D conformal radiotherapy plan in FB, 3D-CRT(FB), 2) and a 3D-CRT plan in breath-hold, 3D-CRT(vmDIBH). The 3D-CRT plans were created using forward planning field-in-field techniques with a mixture of 6 and 10 MV photon beams. Initially, the breast PTV is covered by tangential mediolateral (ML) and lateromedial (LM) fields, including small extra field in field segments to make the dose distribution throughout the PTV more homogeneous, thus making this a forward-planned IMRT technique. For locoregional treatment an anterior-posterior (AP) field (inclined at 15 degrees) is added to cover the nodes in levels I to IV and if necessary to improve target coverage a posterior-anterior beam is added as well. If the IMN are also included in the PTV, the technique is as described previously [Citation11]. The ML field aims at covering the IMN as well. If necessary, the AP field inclined at 15 degrees also includes the IMN nodes. The goal of the 3D-CRT plans is to cover ≥95% of the total and separate PTVs with ≥95% of Dpresc. All treatment plans were performed by a specialized radiation therapist (SH).
For 14 patients, 2 CT scans each, and 3 PTVs each, a total of 84 treatment plans were evaluated and compared. PTV, heart, right (ipsilateral) lung and total lung volumes were reported in FB and for vmDIBH. For the PTV, the fraction of the volume receiving ≥95% of the prescribed dose (V95%), and the near-maximum dose (D1%) which represents the highest dose received by 1% of the volume, were reported. For OAR (lungs, ipsilateral lung, heart, and contralateral breast) the fractional volumes receiving × Gy (VxGy) and mean dose (DMean) were reported and compared. The heart was contoured, including the visceral pericardium, and excluding the inferior- and superior vena cava, ascending- and descending aorta and pulmonary arteries and -veins. Statistical analysis was made using a Wilcoxon signed-rank test, twotailed for each evaluation parameter at a significance level of 0.05.
Results
For the fourteen studied patients, the total lung volume increased from an average of 2760 cc (range 1990 to 3864 cc) in FB to 4447 cc (3167 to 6066 cc) in vmDIBH. The electron density varied between patients and between parts of the lung. In FB the average electron density of lung tissue was around 0.23 g/cm3 and in vmDIBH the average electron density was about 0.16g/cm3. shows the dose- volume parameters for the PTV and normal tissues. shows the MLD for the different techniques for all patients for the total lung. shows the V20Gy for the total lung. For local breast treatment, the reduction in lung dose as a result of breath-hold was very small, with a difference in MLD larger than 1.0 Gy for three of the patients. The reduction was larger for locoregional treatment without IMN, with an average MLD difference of 1.1 Gy. The difference was less than 0.3 Gy for three patients between 1.0 and 2.0 Gy for eight patients, and the maximum MLD reduction in one of the patients was 3.5 Gy. If the IMN were also included, the MLD reduction was 1.7 Gy on average. vmDIBH resulted in an MLD reduction between 1.0 and 2.0 Gy for ten patients, and around 3.0 Gy for two patients. The reduction in MLD and in V20Gy as result of breath-hold was statistically significant for locoregional breast irradiation with and without IMN (p < 0.005).
Figure 1. Mean (total) lung dose for the fourteen patients planned locally, locoregionally without IMN, and locoregionally with IMN, both in FB and in vmDIBH.
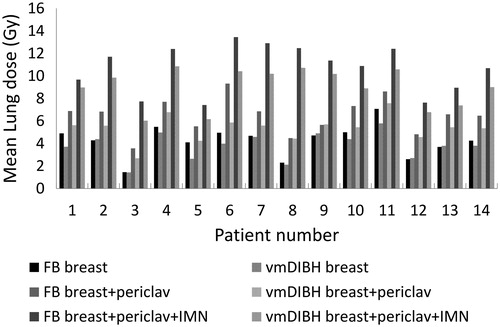
Figure 2. Volume of total lung receiving more than 20 Gy (V20Gy) for the fourteen patients planned locally, locoregionally without IMN, and locoregionally with IMN, both in FB and in vmDIBH.
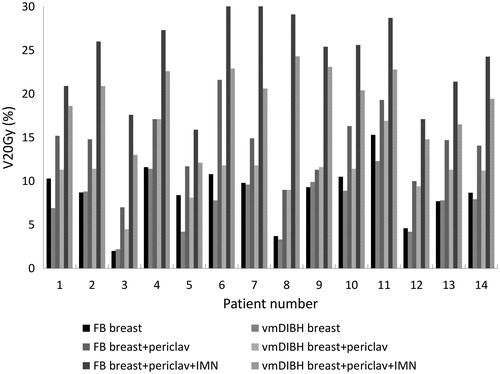
Table 1. Comparison of average dose parameters of 14 right sided breast cancer patients locally irradiated (breast), locoregionally irradiated (Breast + perivlav) and locoregionally irradiated with IMN involved (Breast + periclav + IMN), both in free breathing (FB) and breath-hold (vmDIBH) for the heart, the ipsilateral and total lung and contralateral breast.
As expected, the heart dose was very low for most right-sided breast cancer patients and there was no difference between FB and BH for local breast treatment and for locoregional treatment without IMN. However, if the IMN were also included, the MHD ranged from 0.6 to 4.3 Gy in FB and was reduced from 0.4 Gy to 2.2 Gy in BH. This reduction was not statistically significant (p = 0.07). For ten patients, the reduction in MHD was less than 0.5 Gy, for three patients between 0.6 and 0.75 Gy, and for one patient the reduction was 2.6 Gy.
Discussion
To our knowledge, this is the first paper on the usefulness of breath-hold for right sided breast cancer patients. Two early investigators [Citation6,Citation7,Citation12] included a few right-sided breast cancer patients in their study, but only presented pooled results for all patients. Our data show that breath-hold results in a statistically significant reduction in MLD and in V20Gy for locoregional breast irradiation, especially when the IMN are irradiated. The average reduction in MLD of 1.7 Gy (from 10.7 Gy in FB to 9.0 Gy in vmDIBH) for the fourteen right-sided breast cancer patients in this study is slightly larger than the average reduction of 0.9 Gy (from 8.7 Gy to 7.8 Gy) in our previous study in thirteen left-sided breast cancer patients. Probably this is due to the different anatomy of the right and left lung: the heart is partly situated in the high dose region for left-sided breast cancer patients, especially when the IMN are irradiated. The average MLD reduction of 0.5 Gy for local breast cancer treatments in our study is comparable to the 0.6 Gy reduction reported by Mast et al. [Citation9]. Swanson et al. [Citation8] reported a reduction in MLD of 1.2 Gy, for a mixture of 60% locally and 40% locoregionally treated breast cancer patients, which again is comparable to our total group of locally and locoregionally treated patients.
For four out of fourteen local breast cancer treatments, the MLD was increased by around 0.1 Gy in vmDIBH. For these patients, the MLD in FB was already below average, and although the lung volume also increased for these patients during vmDIBH, perhaps the curvature of the chest wall was slightly unfavourable, resulting in a small, non-relevant increase in lung dose instead of the expected decrease. For the locoregionally treated patients, vmDIBH never resulted an increase in MLD.
The important question remains, whether the reported reduction in lung dose also results in a clinically relevant reduction of pulmonary risks. In the literature, three pulmonary side effects are described: the risk of radiation pneumonitis, decrease in pulmonary function, and the risk of secondary lung cancer [Citation13–25].
A summary of relevant literature on risk of radiation pneumonitis is given in Supplementary material, Table 2. Marks et al. [Citation15] suggest from the QUANTEC information that the risks of symptomatic pneumonitis increases with MLD (total organ) from 5% for a MLD of 7 Gy, 10% for a MLD of 13 Gy and 20% for a MLD of 20 Gy. However, this meta-analysis was especially based on lung cancer patients. The studies for breast cancer patients show lower radiation pneumonitis incidences, e.g., Lind et al. [Citation14] found a radiation pneumonitis rate of 0.9% for locally treated breast cancer patients, and of 4.1% for patients treated with local-regional radiotherapy. They showed a non-statistically significant trend for increasing radiation pneumonitis (RP) with increasing lung volume in the fields. In a later paper, Lind et al. [Citation13] showed that the V20 was, according to multivariate modelling, the most important variable for the occurrence of pulmonary toxicities (p < 0.01). Therefore treatment planning should be aimed at minimizing the volume of lung incidentally irradiated with doses in excess of 20 Gy in adjuvant RT for breast cancer. Smith et al. [Citation16] showed a statistically significant increase in 5 year radiation pneumonitis rate of 0.72% in patients with whole breast irradiation vs 0.12% in patients with brachytherapy partial breast irradiation, also indicating a relation between irradiated lung volume and radiation pneumonitis rate.
Unfortunately, little data is available on non-dosimetric risk factors for developing radiation pneumonitis, such as smoking status, pre-existing lung diseases, chemotherapy before/after radiation treatment and (concurrent) hormonal treatment. Only the study of Lind et al found no confounding effect of concurrent tamoxifen and smoking [Citation13]. These data show that the risk of radiation pneumonitis in locally (around 1%) and locoregionally irradiated patients (around 4%) is low, and is dependent on the volume and dose of irradiated lung tissue. Breath-hold will result in a relatively small decrease in the symptomatic radiation pneumonitis rate: from the studied literature on radiation pneumonitis incidence differences between loco-regional, local and brachytherapy breast treatment combined with the reduction in dose found in our vmDIBH vs FB study we estimate that 1 out of 100 patients will not get radiation pneumonitis as a result of breath-hold.
Two groups investigated the long term effect of local and locoregional irradiation on the pulmonary lung function tests (PFT) with a follow up of respectively 7 [Citation18] and 10 years [Citation25]. Both studies showed an initial decrease in PFTs after 6 months and a recovery at 1 or 2 years. Interestingly, Jaen et al. [Citation18] found a recovery of the PFTs at 7 years and no correlation of the PFT change with age, smoking status, previous chemotherapy and concurrent tamoxifen, while van Erven et al. [Citation25] found a decrease of the PFTs at 10 years. In the multivariate analysis, van Erven et al found three negative predicting factors for worsening PFT: tamoxifen, irradiation of the right side, and an early decrease in forced expiratory 1 second volume and diffusing capacity of the lungs for carbon monoxide. Although there was a change in the PFTs, they were still within the normal range [Citation25]. In lung cancer patients, who were treated with (chemo-)radiation, a change in the density of the Houndfields Units (HU) on CT, before and after 3 months of irradiation, seemed to predict dyspnea grade 2 [Citation17]. This “radionomics” approach could be interesting to investigate in breast cancer patients.
A summary of relevant literature on the risk of secondary lung cancer is given in Supplementary material, Table 3. The data suggest that the risk of secondary lung cancer after breast cancer irradiation mainly increases for (ever) smokers. Grantzau et al. [Citation19] found an excess risk for lung cancer of 17.3%/Gy (this is the reconstructed dose at the center of the lung tumour) for ever smokers (p < 0.005) and of 0.6%/Gy (p > 0.5) for non-smokers. The median time from breast cancer treatment to second lung cancer diagnosis was 12 years (range 1–26 years). They state that “although the absolute risk is relatively low (0.8% in their cohort), the growing number of long-time survivors after breast cancer treatment highlights the need for advances in normal tissue sparing radiation techniques.” The other papers also conclude that never smoking women do not have an increased risk for lung cancer, but smokers do.
Our results show that breath-hold reduces the (mean) lung dose for locoregional treatment, especially in patients with IMN irradiation. Although the risk of lung toxicity and secondary lung cancer is low, the reduction in lung dose will further decrease this risk. We should encourage women to stop smoking to decrease the risk of secondary lung cancer. Future research should focus on identifying risk factors for an increased chance on pulmonary dysfunction or lung cancer, such as age, chemotherapy, hormonal therapy and pre-existing pulmonary diseases. If these risk factors are known, an algorithm can be developed for an individualized planning technique.
With regards to heart toxicity, our data show that for most right sided breast cancer patients, vmDIBH does not result in a relevant dose reduction to the heart. In this study, the reduction in heart dose as a result of breath-hold is clinically relevant [Citation4] for at least one patient (MHD reduction of 2.6 Gy), and possibly also for 3 other studied patients (MHD reduction between 0.6 and 0.75 Gy), since the relative risks of major coronary events increases linearly with the mean heart dose by 7.4% per Gy with no apparent threshold [Citation4]. So, we conclude that for patients with unfavourable anatomy (from this planning study with 4 out of 14 patients, we estimate this to be 10-25% of the patients), the mean heart dose may be reduced between 0.5 and around 2.5 Gy when vmDIBH is applied, especially when the IMN are involved.
This study does not address the possible advantages of breath-hold on liver dose, since the liver was not scanned in our CT scans. The effect of breath-hold on liver dose will be part of a future study.
In a previous study for locoregional radiotherapy including the IMN [Citation11] we demonstrated that adding volumetrtic modulated arc therapy (VMAT) to vmDIBH results in an additional reduction in dose to the heart and the ipsilateral lung when the mean heart dose for the conformal field in field technique with vmDIBH is higher than 3.5 Gy. However, this goes at the cost of a higher dose to the contralateral lung and breast. Our study for left sided breast cancer patients [Citation11], showed that adding VMAT to vmDIBH resulted in a slightly lower dose to the ipsilateral lung (reduction in mean lung dose with about 3.8 Gy from 17.1 Gy to 13.3 Gy on average) but at the same time resulted in a slightly higher dose to the contralateral lung (increase in mean dose from 0.4 to 2.6 Gy on average). The dose to the total lungs was comparable to the dose for the conformal technique. The average dose to the contralateral breast increased from about 0.7 Gy to 2.5 Gy. Since the mean dose to the contralateral lung and breast increased by a few Gy as a result of VMAT, the heart dose will also increase with a few Gy in the case of right-sided breast cancer patients. Therefore, VMAT will not be beneficial for most breast cancer patients. Only for very few patients, with very unfavourable location of the PTV with respect to the heart or a funnel chest ((pectus excavatum) VMAT might result in improved dose delivery for right-sided breast cancer patients.
In conclusion, this study showed that the benefits for lung toxicity reduction as a result of breath-hold, which has become common practice in many institutions for left-sided breast cancer patients, also apply for right-sided breast cancer patients. When lowering the lung dose a lower risk of pulmonary toxicity and secondary lung cancer (in ever-smokers) can be expected. We estimate that radiation pneumonitis can be avoided for 1 out of 100 locoregionally (including IMN) right-sided breast cancer patients using breath-hold. We suggest to routinely use breath-hold not just for left-sided breast cancers but also for all right sided breast cancer patients with locoregional target volume. For some of these patients (we estimate about 10-25% of the patients), breath-hold will have the additional benefit of a significant reduction of the mean heart dose. For locally irradiated right-sided breast cancer patients, the reduction in pulmonary and cardiac risk is hardly clinically significant, and breath-hold techniques are not necessary.
Supp_mat_IONC.zip
Download Zip (67.4 KB)Conflict of interest
None to declare.
References
- Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233–41.
- Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N Engl J Med 2015; 373:317–27.
- Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol 2015;114:3–10.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98.
- Ghobadi G, van der Veen S, Bartelds B, de Boer RA, Dickinson MG, de Jong JR, et al. Physiological interaction of heart and lung in thoracic irradiation. Int J Radiat Oncol Biol Phys 2012;84:e639–46.
- Korreman SS, Pedersen AN, Nottrup TJ, Specht L, Nystrom H. Breathing adapted radiotherapy for breast cancer: comparison of free breathing gating with the breath-hold technique. Radiother Oncol 2005;76:311–18.
- Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA, Wong JW. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys 2003;55:392–406.
- Swanson T, Grills I, Ye H, Entwistle A, Teahan M, Letts N, et. al. Six-Year Experience Routinely Utilizing Moderate Deep Inspiration Breath-hold (mDIBH) for the Reduction of Cardiac Dose in Left-Sided Breast Irradiation for Patients with Early Stage or Locally Advanced Breast Cancer. Am J Clin Oncol 2013;36:24–30.
- Mast M, van Kempen-Harteveld L, Heijenbrok M, Kalidien Y, Rozema H, Jansen W, et al. Left-sided breast cancer radiotherapy with and without breath-hold: Does IMRT reduce the cardiac dose even further? Radiother Oncol 2013;108:248–253
- Hjelstuen MH, Mjaaland I, Vikstrom J, Dybvik KI. Radiation during deep inspiration allows loco-regional treatment of left breast and axillary-, supraclavicular- and internal mammary lymph nodes without compromising target coverage or dose restrictions to organs at risk. Acta Oncol 2012;51:333–44.
- Osman SO, Hol S, Poortmans PM, Essers M. Volumetric modulated arc therapy and breath-hold in image-guided locoregional left-sided breast irradiation. Radiother Oncol 2014;112:17–22.
- Korreman SS, Pedersen AN, Aarup LR, Nottrup TJ, Specht L, Nystrom H. Reduction of cardiac and pulmonary complication probabilities after breathing adapted radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys 2006;65:1375–80.
- Lind PA, Wennberg B, Gagliardi G, Rosfors S, Blom-Goldman U, Lidestahl A, et al. ROC curves and evaluation of radiation-induced pulmonary toxicity in breast cancer. Int J Radiat Oncol Biol Phys 2006;64:765–70.
- Lind PA, Rosfors S, Wennberg B, Glas U, Bevegard S, Fornander T. Pulmonary function following adjuvant chemotherapy and radiotherapy for breast cancer and the issue of three-dimensional treatment planning. Radiother Oncol 1998;49:245–54.
- Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70–6.
- Smith GL, Xu Y, Buchholz TA, Giordano SH, Jiang J, Shih YC, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA 2012;307:1827–37.
- De Ruysscher D, Sharifi H, Defraene G, Kerns SL, Christiaens M, De Ruyck K, et al. Quantification of radiation-induced lung damage with CT scans: the possible benefit for radiogenomics. Acta Oncol 2013;52:1405–10.
- Jaen J, Vazquez G, Alonso E, De Las Penas MD, Diaz L, De Las Heras M, et al. Long-term changes in pulmonary function after incidental lung irradiation for breast cancer: a prospective study with 7-year follow-up. Int J Radiat Oncol Biol Phys 2012;84:e565–70.
- Grantzau T, Thomsen MS, Vaeth M, Overgaard J. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother Oncol 2014;111:366–73.
- Grantzau T, Overgaard J. Risk of second non-breast cancer after radiotherapy for breast cancer: A systematic review and meta-analysis of 762,468 patients. Radiother Oncol 2015;114:56–65.
- Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999;45:323–29.
- Kaufman EL, Jacobson JS, Hershman DL, Desai M, Neugut AI. Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. J Clin Oncol 2008;26:392–98.
- Prochazka M, Hall P, Gagliardi G, Granath F, Nilsson BN, Shields PG, et al. Ionizing radiation and tobacco use increases the risk of a subsequent lung carcinoma in women with breast cancer: case-only design. J Clin Oncol 2005; 23:7467–74.
- Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer 2013;108:179–82.
- Erven K, Weltens C, Nackaerts K, Fieuws S, Decramer M, Lievens Y. Changes in pulmonary function up to 10 years after locoregional breast irradiation. Int J Radiat Oncol Biol Phys 2012;82:701–7.

