Abstract
Background Predictive markers and prognostic models are required in order to individualize treatment of recurrent glioblastoma (GBM) patients. Here, we sought to identify clinical factors able to predict response and survival in recurrent GBM patients treated with bevacizumab (BEV) and irinotecan.
Material and methods A total of 219 recurrent GBM patients treated with BEV plus irinotecan according to a previously published treatment protocol were included in the initial population. Prognostic models were generated by means of multivariate logistic and Cox regression analysis.
Results In multivariate analysis, corticosteroid use had a negative predictive impact on response at first evaluation (OR 0.45; 95% CI 0.22–0.93; p = 0.03) and at best response (OR 0.51; 95% CI 0.26–1.02; p = 0.056). Three significant (p < 0.05) prognostic factors associated with reduced progression-free survival and overall survival (OS) were identified. These factors were included in the final model for OS, namely corticosteroid use (HR 1.70; 95% CI 1.18–2.45; p = 0.004), neurocognitive deficit (HR 1.40; 95% CI 1.04–1.89; p = 0.03) and multifocal disease (HR 1.56; 95% CI 1.15–2.11; p < 0.0001). Based on these results a prognostic index able to calculate the probability for OS at 6 and 12 months for the individual patient was established. The predictive value of the model for OS was validated in a separate patient cohort of 85 patients.
Discussion and conclusion A prognostic model for OS was established and validated. This model can be used by physicians to risk stratify the individual patient and together with the patient decide whether to initiate BEV relapse treatment.
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults. Despite aggressive standard treatment, comprising maximal surgical resection, radiation therapy and concomitant and adjuvant temozolomide, newly diagnosed patients have a median overall survival (OS) of less than 15 months [Citation1]. At tumor recurrence no standard treatment is available and most known options have limited clinical effect.
Several phase II studies of recurrent GBM patients treated with bevacizumab (BEV) combination therapy have shown high response rates [Citation2,Citation3] and patients who achieve response have an improved OS as well as quality of life [Citation4–6]. Consequently, BEV combined with irinotecan (IRI) has been among the most frequently used treatments for recurrent GBM over the last years. Recently, the BELOB trial suggested that the combination of BEV with lomustine is superior to either agent used alone [Citation7]. This is the first randomized study to indicate a survival benefit in recurrent GBM treated with BEV containing therapy. A confirmative phase III trial is awaited.
In non-BEV regimens used in recurrent GBM, the following prognostic factors have been associated with OS: Age [Citation8], WHO performance status (PS) [Citation8,Citation9], corticosteroids [Citation8,Citation9], tumor size [Citation9], multifocal disease [Citation9], and frontal tumor location [Citation9]. However, studies of these factors in recurrent GBM patients treated with a BEV containing therapy have shown inconsistent results [Citation10–12]. More importantly, no validated predictive baseline markers associated with clinical durable response to BEV containing therapy have been identified.
The aim of this study was to identify prognostic and potentially predictive clinical baseline factors associated with response, progression-free survival (PFS) and OS in recurrent GBM patients treated with BEV plus IRI.
Materials and methods
Patients
This retrospective study was conducted in accordance with the Helsinki Declaration and was approved by the Danish Data Protection Agency (2006-41-6979).
Population 1 (initial cohort): By using a pharmacy database and a pathology database, we identified all patients with a pathologically confirmed diagnosis of GBM (WHO grade IV) who were treated at recurrence with BEV plus IRI between May 2005 and December 2013 at Rigshospitalet. During this period, BEV 10 mg/kg and IRI 125 mg/m2 every two weeks could be prescribed to all recurrent GBM patients older than 18 years in WHO PS 0-2 at baseline. Patients had to have measurable progressive disease by contrast-enhanced magnetic resonance imaging (MRI) after standard therapy and be at least four weeks from prior chemotherapy and three months from completion of radiotherapy. For patients who had undergone relapse surgery a post-surgical MRI was performed prior to treatment initiation. Detailed inclusion and exclusion criteria are described in a previously published treatment protocol [Citation5].
Population 2 (validation cohort): From a previously published cohort of 292 high-grade gliomas diagnosed between January 2005 and December 2009 at Odense University Hospital [Citation13], we identified all patients with recurrent GBM treated with BEV and IRI.
Clinical follow-up
Clinical follow-up was performed every four weeks and MRI every eight weeks. MRI T1 and T2 sequences were employed, and in recent years the T2/fluid-attenuated inversion recovery (FLAIR) sequence was added. Treatment response and date of progression were evaluated (investigator assessment) using the Macdonald criteria [Citation14]. In the more recent cases evaluated by the T2/FLAIR sequence, response and date of progression were retrospectively reviewed (by T.U., V.A.L.) based on Revised Assessment in Neuro-Oncology (RANO) criteria [Citation15] and a high concordance with the initial evaluation was observed.
Statistical analysis
Response was estimated by employing logistic regression and the results presented by odds ratios with 95% confidence intervals (95% CI) and the area under the receiver operating characteristic curve. Survival probabilities were estimated with the Kaplan-Meier method. Multivariable analysis included covariates with p-value less than 10%. The Cox proportional hazards model was used for multivariable analysis. Assessment of the model assumptions was done using martingale residuals. The results are presented by hazard ratios with 95% CI and the concordance index (C-index) as a measure of discrimination [Citation16]. A prognostic index for OS was developed based on the final multivariate analysis. The linear combination of the chosen covariates were assessed for 6- and 12-month survival using logistic regression and presenting the results by the sensitivity, specificity as well as the area under the receiver operating characteristic curve. The predictor for response, PFS and OS from the training set was calculated for the validation study and tested in this. p-values <0.05 were considered significant. Calculations have been performed using SPSS (v19.0, IBM Corp., Armonk, NY, USA), R (R Development Core Team, Vienna, Austria, http://www.R-project.org) and SAS (v9.3, SAS institute, Cary, NC, USA) software.
Results
Patient characteristics
Population 1: A total of 219 recurrent GBM patients (73 women, 146 men) treated with BEV plus IRI were identified. Patient characteristics are summarized in (full list according to screened factors, see Supplemental , available online at //www.informahealthcare.com). After progression on BEV and IRI therapy, 22 patients underwent resurgery and 19 patients received various types of experimental treatments. Twenty patients were alive at the end of follow-up of whom seven had not progressed (median follow-up = 7.4 months, range 0.3–69 months).
Table I. Patient characteristics.
Population 2: A total of 85 patients were identified and included in the validation dataset. Characteristics are listed in . Two patients were alive and all had progressed at the end of follow-up (median follow-up = 7.8 months, range 0.9–76 months).
Response, PFS and OS, Population 1
A response was found in 66 of 219 patients (30%; 95% CI 24–36). Twenty-two patients were non-evaluable at first response evaluation due to early progression, death or toxicity. Of the 197 evaluable patients, 66 patients were classified as responders (complete or partial response) and 131 patients as non-responders (stable or progressive disease). Among the responders 54 patients (82%) achieved response at first treatment evaluation. In univariate analysis, factors associated (p < 10%) with a reduced chance of response at first response evaluation and at time of best response were: WHO PS 2 versus 0, corticosteroid use, higher corticosteroid dose and increasing tumor size.
Median PFS was 5.0 months (95% CI 4.2–5.3) and 6-month PFS was 33.5% (95% CI 27.3–39.8). In univariate analysis, factors associated with reduced PFS were: Increasing age (p = 0.06), WHO PS 2 vs. 0 (p = 0.009), multifocal disease (p = 0.002), corticosteroid use (p = 0.01), higher corticosteroid dose (p = 0.005), increasing tumor size (p = 0.05), neurological objective deficit (p = 0.05), neurocognitive deficit (p = 0.02), aphasia (p = 0.01), hemiparesis (p = 0.03) and ataxia (p = 0.009).
The median OS for all subjects was 7.5 months (95% CI 6.7–8.2). OS at 6 and 12 months were 63.8% (95% CI 57.4–70.2%) and 23.5% (95% CI 17.8–29.3%). Factors associated with decreased OS in univariate analysis were: WHO PS 1 versus 0 (p = 0.01), WHO PS 2 versus 0 (p = 0.0005), multifocal disease (p < 0.00001), corticosteroid use (p = 0.0002), higher corticosteroid dose (p < 0.00001), increasing tumor size (p = 0.009), neurological objective deficit (p = 0.02), neurocognitive deficit (p = 0.01), hemiparesis (p = 0.02), ataxia (p = 0.02) and aphasia (p = 0.05).
Multivariate analysis and prognostic models, Population 1
summarizes the multivariate analyzes and prognostic models for response and survival endpoints. Corticosteroid dose (continuous) and corticosteroid use added equal predictability to the models for response, PFS and OS and in order to simplify the models it was decided to include only corticosteroid use. Use of corticosteroid was inversely related to response and the only predictor to have a significant impact on the likelihood of achieving a response at first evaluation (OR 0.45; 95% CI 0.22–0.93; p = 0.03) and at best response (OR 0.51; 95% CI 0.26–1.02; p = 0.056). Tumor size and WHO PS were not associated with response (p > 0.20). Nevertheless, it was decided to adjust the models for WHO PS along with the prognostic factors identified below. The model for response at first evaluation had a C-index of 0.66 and the model for best response had a C-index of 0.63.
Table II. Multivariate analysis of response at first evaluation, best response, progression-free survival and overall survival (Population 1).
Multivariate analysis of PFS identified neurocognitive deficit (HR 1.33; 95% CI 1.00–1.77; p = 0.049), multifocal disease (HR 1.56; 95% CI 1.15–2.11; p = 0.004) and corticosteroid use (HR 1.42; 95% CI 1.00–2.00; p = 0.049) as significant prognostic factors associated with an increased risk of progression. Remaining factors identified in univariate analysis were not significantly associated with PFS. However, it was decided to keep WHO PS in the model based on the reasons described above. The C-index for the model was 0.63.
Of factors identified by univariate analysis, three were found significantly associated with reduced OS by multivariate analysis when adjusted for WHO PS: neurocognitive deficit (HR 1.40; 95% CI 1.04–1.89; p = 0.029), multifocal disease (HR 1.87; 95% CI 1.37–2.56; p < 0.0001) and corticosteroid use (HR 1.70; 95% CI 1.18–2.45; p = 0.004). The C-index was 0.64.
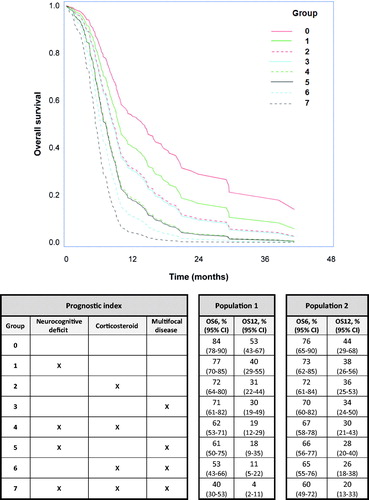
shows the estimated survival curves based on the combinations of the three significant covariates included in the final model for OS. The median OS for the best group (negative for all three covariates) was 13.8 months and 5.3 months for patients in the group with the poorest signature (positive for all three covariates). The estimated 6- and 12-month OS corresponding to the eight possible combinations of the three markers are shown in . The area under the curve (AUC) for 6-month survival was 0.67, with 51% sensitivity at 75% specificity and for 12-month survival, the AUC was 0.71 with 55% sensitivity at 75% specificity.
Validation of prognostic models
Application of the predictor established in Population 1 for best response to the validation set (Population 2) showed a non-significant association (p = 0.22) with a C-index of 0.58. The results of applying the predictor for PFS to the validation study showed a significant association (p = 0.01) with a C-index of 0.59. OS in the validation study demonstrated a significant association to the predictor estimated from the training set (p = 0.03) with a C-index of 0.62. Collectively, the predictor for response was not validated, while the model for PFS and OS was validated. The estimated OS probabilities for 6 and 12 months for the validation study using the predictor from the training set are shown in .
Discussion
In this retrospective study of 219 patients with recurrent GBM treated with BEV plus IRI we found that prognostic factors were: Corticosteroid use, multifocal disease and neurocognitive deficit. Based on these factors a prognostic model for OS was established and validated in a cohort of 85 patients from another center.
The two cohorts included in this study differed in several patient characteristics. However, as median PFS and OS of 5.0 months and 7.5 months in the initial cohort were comparable to the validation cohort and other retrospective studies [Citation10,Citation12], we conclude that our cohorts are representative for the general population of recurrent GBM patients treated at different centers outside clinical trials.
In the initial cohort the response rate was 30% which was significantly lower than in the validation dataset. Response assessment is susceptible to considerable inter-observer variability and this may explain most of the difference in our observed response rates and why we were not able to predict response in the validation cohort. In the initial cohort corticosteroid use was the only predictor inversely associated with response and prolonged PFS and OS. Corticosteroid use is a prognostic factor in recurrent GBM [Citation8,Citation9,Citation11] and consequently may reflect a subgroup of patients less likely to achieve early benefit from BEV containing therapy in terms of response. In addition, corticosteroid dose changes are known to influence contrast enhancement, though the effect is considered transient (<2 weeks) [Citation17]. Also worth considering is a possible corticosteroid-like effect of BEV influencing the contrast enhancement. Hypothetically, the use of corticosteroids at baseline may diminish the BEV-related effect on contrast enhancement at subsequent scans, reducing the likelihood of a radiological response. Lastly, use of corticosteroids was found significantly associated with a higher risk of developing severe (grade ≥3) lymphocytopenia. Whether this or other immunosuppressive effects of corticosteroids interfere with the efficacy of BEV remains unanswered.
In addition to corticosteroid use, neurocognitive deficit and multifocal disease were identified as independent poor prognostic factors for both PFS and OS. All three have previously been reported to affect survival in non-BEV-treated recurrent GBM patients [Citation8,Citation9,Citation18]. In a recently published study on BEV-treated patients, corticosteroid use was associated with reduced PFS and OS [Citation11]. To our knowledge, no other study has identified multifocal disease or neurocognitive deficit as being prognostic in BEV-treated recurrent GBM patients. In contrast to our expectations, and to previous studies [Citation8,Citation9,Citation11,Citation12], PS was not an independent prognostic factor. This might be due to a low number of patients with poor PS, which was highly associated with the use of corticosteroids. Consequently, the statistical power may not be adequate to detect an association between PS and survival endpoints.
In the present study we developed and validated prognostic models for GBM patients who receive BEV plus IRI at recurrence. Three risk factors predictive of early progression and mortality were identified: Neurocognitive deficit, corticosteroid use and multifocal disease. The prognostic model can help physicians to objectively inform patients about their prognosis and collaboratively decide whether the patient should or should not initiate BEV relapse treatment.
Suppl_Table_S1.doc
Download MS Word (84 KB)Declaration of interest
The authors have no conflicts of interest to declare.
Funding
Financial support was kindly provided by Rigshospitalet, the Danish Cancer Society, Doctor Sophus Carl Emil Friis and Wife Olga Doris Friis’ Grant and the I.M. Daehnfeldt Foundation. An unrestricted grant was provided from Roche A/S (Hvidovre, Denmark) and the company was not involved in interpretation of the results of this study or in presentation of the data.
References
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96.
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733–40.
- Vredenburgh JJ, Desjardins A, Herndon JE, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007;25:4722–9.
- Henriksson R, Asklund T, Poulsen HS. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: a review. J Neurooncol 2011;104:639–46.
- Poulsen HS, Grunnet K, Sorensen M, Olsen P, Hasselbalch B, Nelausen K, et al. Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol 2009;48:52–8.
- Prados M, Cloughesy T, Samant M, Fang L, Wen PY, Mikkelsen T, et al. Response as a predictor of survival in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol 2011;13:143–51.
- Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 2014;15:943–53.
- Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol 2007;25:2601–6.
- Gorlia T, Stupp R, Brandes AA, Rampling RR, Fumoleau P, Dittrich C, et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer 2012;48:1176–84.
- Chen C, Huang R, Maclean A, Muzikansky A, Mukundan S, Wen PY, et al. Recurrent high-grade glioma treated with bevacizumab: prognostic value of MGMT methylation, EGFR status and pretreatment MRI in determining response and survival. J Neurooncol 2013;115:267–76.
- Duerinck J, Clement PM, Bouttens F, Andre C, Neyns B, Staelens Y, et al. Patient outcome in the Belgian medical need program on bevacizumab for recurrent glioblastoma. J Neurol 2015;262: 742–51.
- Tabouret E, Barrie M, Thiebaut A, Matta M, Boucard C, Autran D, et al. Limited impact of prognostic factors in patients with recurrent glioblastoma multiforme treated with a bevacizumab-based regimen. J Neurooncol 2013;114:191–8.
- Dahlrot RH, Kristensen BW, Hjelmborg J, Herrstedt J, Hansen S. A population-based study of high-grade gliomas and mutated isocitrate dehydrogenase 1. Int J Clin Exp Pathol 2013;6:31–40.
- Macdonald DR, Cascino TL, Schold SC JrJr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8:1277–80.
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963–72.
- Harrell FE JrJr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15: 361–87.
- Watling CJ, Lee DH, Macdonald DR, Cairncross JG. Corticosteroid-induced magnetic resonance imaging changes in patients with recurrent malignant glioma. J Clin Oncol 1994;12:1886–9.
- Meyers CA, Hess KR, Yung WK, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol 2000;18:646–50.