Abstract
Background We compared the development of adverse effects and psychosocial measures from baseline to 36-month follow-up in patients with prostate cancer (T1-3 M0) referred to our department for definitive radiotherapy encompassing the prostate and pelvic lymph nodes (RAD + IMRT) or radiotherapy to the prostatic gland only (RAD), applied with standard adjuvant androgen deprivation (AD) in all patients. Few studies have explored the impact of fatigue on patients’ reported quality of life (QoL) after combined therapy for prostate cancer. Material and methods The 206 consecutive eligible men (RAD + IMRT = 64 and RAD = 142) completed the UCLA-PCI questionnaire for adverse effects at baseline, 12, 24, and 36 months. QoL, anxiety and depression, and fatigue were rated at the same time points. Between-group and longitudinal within-group changes at different time points were reported. At 36 months variables associated with fatigue were analyzed with regression analyses. Results Our main novel finding is the long-term high level of fatigue and high prevalence of chronic fatigue, affecting patients receiving radiotherapy combined with long-term AD. Except for urinary bother in the RAD + IMRT group all functions and the other bothers mean scores were significantly worse at 36 months compared to baseline. In multivariable analyses only physical QoL remained significantly associated with fatigue at 36-months follow-up. Conclusions Fatigue and impaired QoL in patients considered to curative irradiation with long-term AD should be addressed when counseling men to combined treatment.
Intensity-modulated radiotherapy (IMRT) has opened the field of radiotherapy for extended radiation volumes achieving isodose effectiveness to whole pelvis radiation without significantly increasing the damage to normal tissue in patients treated for prostate cancer (PCa) [Citation1–3]. In the present study of adverse effects the IMRT regime consisted of two radiation techniques, first an intensity-modulated regime encompassing the pelvic lymph node instead of the whole pelvis, the prostatic gland and seminal vesicles, and second, a boost volume covering the prostate with or without the seminal vesicles applying a four-field box technique (RAD regime).
Due to modern imaging facilities and higher dose conformity applied to the in field-volumes, short-term physician and patient-reported toxicity is encouraging [Citation1,Citation2,Citation4,Citation5]. By using validated instruments consisting of several domains, such as the UCLA-PCI, we addressed the impact of sexual, urinary and bowel function and as well bother and their relevance of possible distress to the patient’s fatigue and health-related quality of life (QoL). In addition, in recent reports acute toxicity (up to 24-month follow-up) function and bother scores were improved applying IMRT [Citation6,Citation7]. The issue of fatigue after unspecified RAD has previously been reported by our group, finding increased prevalence of chronic fatigue after RAD both with and without neo-adjuvant androgen deprivation therapy (ADT). However, these results were based on national sampling of PCa patients [Citation8,Citation9]. The relationship between fatigue, adverse effects, and QoL has not been addressed in depths in men with locally advanced PCa with adequate instruments. Data on long-term toxicity when combining increased irradiated volumes with long-term ADT in patients perceived adverse effects are strongly needed.
In the present prospective follow-up study, we compared patients treated for locally advanced PCa by either RAD + IMRT or RAD from pretreatment (baseline) to 36-month follow-up both cross-sectionally and longitudinally on: 1) patient-assessed adverse effects (function and bother); 2) patient-assessed fatigue, QoL, and anxiety and depression; and 3) what independent variables are associated with total fatigue and chronic fatigue at 36 months as dependent variables.
Patients and methods
Group characteristics
This study only concerns the patients treated with RAD + IMRT (n = 64) or RAD (n = 142) who completed the questionnaires at all time points (baseline, and 12-, 24-, and 36-month follow-up). All patients (both RAD + IMRT and RAD) started neo-adjuvant ADT six months prior to radiotherapy, and this treatment was continued to a maximum of 30 months in some patients with pN + and a prognostic very high-risk profile. We applied a three-month depot injection with gosereline (Zoladex® 10.8 mg sc).
IMRT + RAD patients
Initially 120 patients were eligible, but 30 were omitted due to protocol deviations. At the 36-month follow-up 64 of 90 patients (71% of baseline sample) had responded at all time points. The protocol was started in November 2005 and closed in August 2010. In addition to the anatomical grading using the TNM/UICC stage classification [Citation10], the inclusion criteria were: age <75 years, no previous invasive cancer, initial PCa diagnosis made during the last six months, pN + M0 or a calculated N + risk ≥15% using the Memorial Sloan-Kettering Cancer Center nomogram [Citation11], and prognostic high-risk disease defined by D’Amico’s classification [Citation12]. During the recruitment period a trend towards radiographic N-assessment was seen due to limited and inappropriate lymph node dissection when applying lymphadenectomy [Citation13].
The clinical target volume for the pelvic nodes was delineated by contouring a 0.7 cm radial area around the pelvic iliac vessels and adding a margin to planning target volume [Citation14]. The medial portion of the pre-sacral nodal area was left out in the delineation of lymph nodes, aiming to spare the recto-sigmodeum, otherwise the countering was much alike the recommendations published by the Radiation Therapy Oncology Group Web site (www.rtog.org.).
The rectum was delineated from the anus to the rectosigmoid flexure. In the approved IMRT protocol predefined protocol-stated dose constraints to the organs at risk were mandatory. The following dose constraints were used: ≥70 Gy to maximum 30% and ≥60 Gy to 50% of the volume of the rectum, respectively, and ≥70 Gy to maximum 20% and ≥65 Gy to maximum 50% of the volume of the bladder, respectively.
Inverse planning software was applied (Nucleotron, Veenendal, The Netherlands). Treatment plans were generated by seven coplanar fields to the delineated pelvic structures up to a total dose of 50 Gy encompassing the prostate and vesiculae seminales (target volumes; PV), by use of 15-MV photon beams. Radiation of the boost volume (24 Gy to the seminal vesicles and the prostate for T3b; 24 Gy to the prostate for ≤ T3a) was done by a four-field box technique. Patients were instructed to empty the rectum and keep the bladder filled during the course of radiotherapy. The radiation dose was described at the isocenter according to the International Commission on Radiation Units and Measurements Reports 62 (ICRU Report 62, www.icru.org).
RAD patients
Between December 2004 and July 2007 all patients who received curative treatment for PCa at our department were invited to take part in a longitudinal study focused on adverse effects. For the present study the sub-sample of patients (n = 180) with high-risk disease according to D’Amico treated with RAD was used. The inclusion criteria were similar to those described for the RAD + IMRT patients. In the RAD sample 142 patients of 180 (79% response rate) completed the questionnaires at all time points.
All patients were treated with conformal four-field box radiation technique to the target volumes (50 Gy to the seminal vesicles and prostate and boost 24 Gy to the prostate for ≤ T3a, or 24 Gy to the prostate and seminal vesicles for T3b) and the same planning instructions as applied for IMRT were used.
Normative data
A previous study of Norwegian population sample provided us with normative data on fatigue. In men aged 60 years or more the mean scores were: mental fatigue 4.5 (SD 1.2), physical fatigue 8.4 (SD 3.2), and total fatigue 12.9 (SD 3.8), and caseness of chronic fatigue 16.8% [Citation15].
Questionnaires and variable descriptions
The following questionnaires were completed by all patients at all time points.
The UCLA-PCI. The UCLA-PCI is an established instrument with a time frame of the last four weeks [Citation16], and the patients report on physiological functions (several items) within each of the urinary, bowel, and sexual domains after treatment for PCa. Bother concerns the degree of worry or problem that the patients attach to their physiological functions. The scoring alternatives for both function and bother within all three domains are: “no problem” (5), “very small problem” (4) “small problem” (3), “moderate problem” (2) and “big problem” (1). The scorings were converted to scales ranging from 100 (no bother, maximum function) to 0 (maximum bother, minimum function) according to established algorithms. Functions and bother were considered as continuous variables.
The Hospital Anxiety and Depression Scale (HADS). The HADS consists of seven items each on the anxiety and depression sub-scales. The item scores range from 0 (not present) to 3 (highly present) last week, so the sub-scale scores range from 0 (low) to 21 (high) [Citation17].
The Fatigue Questionnaire (FQ). Several instruments are available for self-rating of fatigue, however, Norwegian clinical oncological research have a tradition for using the FQ, perhaps due to the access of population-based scores [Citation15]. The FQ contains 11 items covering four mental and seven physical fatigue items the last four weeks each rated from 0 (as before) to 3 (very much more). The mental fatigue score ranges from 0 to 12 and the physical score from 0 to 21, with higher scores implying more fatigue. The total fatigue score represents the sum of these scale scores and ranges from 0 to 33. Chronic fatigue is defined as a high level of total fatigue for at least six months according to an established algorithm [Citation18].
The UCLA-PCI, the HADS and the FQ showed adequate internal consistency with Cronbach’s coefficient ≥0.65 at baseline.
The Short Form 12 (SF-12). The SF-12 questionnaire covers physical and mental QoL, expressed as the Physical (PCS) and Mental Composite Summary scores (MCS). Based on T-transformations of the scorings, the mean PCS and MCS scores in the general Norwegian male population are 50 points. One standard deviation covers 10 points [Citation19].
Current paired relationship meant being married or cohabiting versus non-paired relationships, level of basic education was dichotomized into low (<13 years) and high (≥13 years) and current work status was dichotomized into being in paid work versus not in paid work. Somatic comorbidity was recorded if one or more of the diseases covered by the Medical History Checklist of the UCLA-PCI were present at baseline [Citation20].
Statistics
Cross-sectional differences between the IMRT and RAD groups were analyzed with t-tests for continuous variables, and in case of skewed distributions, the Mann-Whitney U-test was used. Categorical differences were analyzed with χ2-tests. As the IMRT group contained significantly more severe cases of PCa (), all between-group analyses of adverse effects and psychosocial measures were adjusted for clinical tumor stage, Gleason score, and presence of lymph node by multivariate linear regression. For longitudinal analyzes paired sample t-tests were used to analyze the changes in the UCLA-PCI, the FQ, the HADS, and the SF-12 scores between all four time points. Comparisons with normative data were performed with one sample t-test for continuous data and with χ2-tests for categorical ones.
Table I. Demographic and clinical characteristics at baseline of the IMRT sample (N = 64) and the RAD sample (N = 142) that completed questionnaires at 36 months.
We used linear regression analyses to examine the relation between independent variables and total fatigue as dependent variable at 36 months. The strengths of associations in linear regression were expressed as beta (B) and standardized beta (β) values. We used logistic regression analyses to examine the relation between independent variables and chronic fatigue cases as dependent variable at 36 months. The strengths of associations in logistic regression were expressed as odds ratios (ORs) with 95% confidence intervals (95% CI) as appropriate.
A p-value < 0.05 was regarded as statistically significant, and all tests were two-sided. The data were analyzed with the IBM SPSS Statistic software for PC version 21.0.
Ethical approval
Both the RAD + IMRT and the RAD protocols were approved by the local hospital ethical board and were approved by the Regional Committee for Medical and Health Research Ethics of South-East Norway. All patients provided written informed consent.
Results
Attrition analyses
The non-responders of both the RAD and RAD + IMRT groups at 36 months were compared to the responders on all questionnaire data and clinical and demographic data at baseline, and no significant differences were observed between the non-responders and responders in any of the groups (Data not shown).
Group comparisons at baseline
No significant between-group differences were observed at baseline socio-demographic, somatic, or lifestyle variables. Although all patients belonged to the D’Amico high-risk pretreatment group, the IMRT + RAD group had clinically significant more patients with clinical T3-T4 tumors, with higher Gleason scores, and infiltrated lymph nodes than the RAD group (), and as mentioned under Statistics we adjusted our between-group analyses for these clinical differences.
Function and bother scores
When comparing the IMRT + RAD and the RAD groups, urinary function was significantly better in the latter group at all time points, and so was also bowel function at 36 months (). Urinary bother at baseline and bowel bother at 12 months, and urinary bother at 36 months were significantly less in the RAD group.
Table II. Mean function and bother scores of IMRT + RAD and RAD patients at baseline, 12-, 24-, and 36-months follow-up.
Significant changes over the observation period were found for all functions and bothers in both the RAD + IMRT and the RAD groups mostly with the pattern that the baseline function and bother mean values were significantly higher than those later on during the observation period. Except for urinary bother in the RAD + IMRT group all functions and the other bothers mean were significantly worse at 36 months compared to baseline.
Psychosocial scores
Comparing the RAD + IMRT and RAD groups significantly higher levels of anxiety were observed for the former group at baseline and 12 months. The mental QoL was significantly better in the RAD group at 24 and 36 months, while the physical QoL group also showed that pattern at 24 months. No significant between-group differences were observed for any of the fatigue measures (). To support reading of the between-group fatigue findings over time, they are illustrated by .
Figure 1. (A) Proportion of cases with chronic fatigue in the IMRT and RAD groups at various time points. There are no significant groups differences. All prevalences except IMRT at baseline are significantly larger than normative prevalence (16.8%). (B). Mean mental fatigue scores in the RAD groups at various time points. There are no significant between-group differences. (C). Mean physical fatigue scores in the IMRT and RAD groups at various time points. There are no significant between-groups differences. (D). Mean total fatigue scores in the IMRT and RAD groups at various time points. There are no significant between-groups differences.
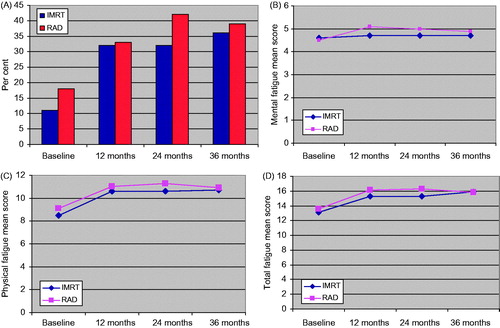
Table III. Mean function and bother scores of IMRT + RAD and RAD patients at baseline, 12-, 24-, and 36-months follow-up.
No significant changes over time were observed for the level of depression in any of the treatment groups. The level of anxiety increased significant from baseline to 36 months in the RAD + IMRT group, while the opposite development was the case in the RAD group. In contrast the level of total fatigue increased significantly from baseline to 36-month follow-up in both groups. In the RAD + IMRT group the level of physical QoL was significantly reduced from baseline to 36 months, while no such change was observed in the RAD group. Mental QoL did not change from baseline to 36 months in the RAD + IMRT group, while there was a significant increase over time in the RAD group ().
Regression analyses
At 36 months in bivariate analyzes bowel function, bowel bother, urinary bother, HADS Depression, MCS-12 and PCS-12 were all significantly associated with both the total fatigue score and the caseness of chronic fatigue. In the multivariate analyses only PCS-12 remained significantly associated with both these dependent variables ().
Table IV A and B. Bivariate and multivariable linear regression analyzes at 36-months follow-up with total fatigue and caseness of chronic fatigue as dependent variables (N = 206).
In addition comorbidity, urinary function, and HADS Anxiety were significantly associated with fatigue total score in bivariate analyses. Correspondingly sexual bother was significantly associated with chronic fatigue.
Comparison with normative data
Compared to the 16.8% prevalence of chronic fatigue among men aged ≥60 years, the RAD group had a significantly higher prevalence at all time points, while the IMRT group showed the same at all follow-up time points. Mental fatigue was significantly higher than normative values in IMRT group at all follow-up time points, while the same was observed for RAD group all time point regarding physical and mental fatigue. In the IMRT group physical and total fatigue means scores were significantly above the normative values at all follow-up time points ().
Discussion
Main findings
To the best of our knowledge our report describes fatigue as a significant contributor to QoL of men with PCa after RAD + IMRT. Moreover, it is the first patient-based study of fatigue in men with locally advanced PCa including the pelvic lymph nodes by modern IMRT and long-term ADT. The RAD + IMRT group showed significantly poorer urinary function at all time points and poorer bowel function at 12 and 36 months compared to the RAD group and the patients of both groups had long-term ADT for aggressive PCa. In both groups both functions and bothers generally were poorer at 36-month follow-up compared to baseline. The same patterns were observed for anxiety, mental and physical QoL, physical and total fatigue as well as chronic fatigue. Compared to the prevalence of 16.8% of chronic fatigue in a Norwegian population sample of men aged ≥60 years, 36% in the IMRT + RAD and 39% in the RAD group are definitely higher. At 36 months bowel function, bowel bother, urinary bother, HADS Depression, MCS-12 and PCS-12 were all significantly associated with the total fatigue score and chronic fatigue in bivariate analyzes. In multivariate analyzes only PCS-12 remained significantly associated with both these dependent variables
Comparison with previous studies
The onset of fatigue has been reported during the course of ADT with radiotherapy in three randomized trials [Citation21–23]. However, fatigue was not prolonged. Our findings are in strong contrast to published reports where most patients recovered from fatigue over time after radiotherapy or discontinuation of ADT [Citation6,Citation24]. This might in part be due to the low statistical power in some studies and limited questionnaires (PR-25, EORTC-30) not covering the issue of fatigue profoundly compared to our assessment applying the FQ instrument.
Self-limiting fatigue not related to urinary, intestinal or sexual dysfunction has been described in both radiotherapy and ADT series [Citation25,Citation26]. Thus, the increased urinary bother observed in the RAD + IMRT group and the findings of decreased bowel function and bowel bother in the regression analyses may hint to a contributing factor establishing the symptom cluster of total fatigue, the main prevalent symptom in this study. These findings suggest that the presence of total fatigue may be partly influenced by the sequelae of combined treatment as otherwise presumed [Citation23]. Interestingly, comparing our finding on fatigue to Norwegian normative data also patients in the RAD cohort had substantially impaired QoL [Citation8,Citation9,Citation15].
Similar to our study, ADT and its impact on QoL has been linked to fatigue in men with locally advanced PCa [Citation25]. That study found more fatigue in the leuprolide group compared to the bicalutamid group at 12 months. The difference in observed fatigue remained detectable over the study period and may be related to the extended irradiated volume and the duration of ADT in our RAD + IMRT cohort.
Thus, in the absence of prospective studies for men with locally advanced PCa showing a clear outcome benefit of pelvic radiation, diminishing treatment-related side effects should be a top priority. In this line, technological improvements such as shown for image-guided irradiation with daily field control by fiducial markers [Citation27] and strategies addressing potential systemic treatments with a lower toxicity profile substituting partly ADT should be sought.
Prospective randomized trials have reported improved survival outcome when combining primary radiotherapy with long-term and even life-long ADT [Citation28,Citation29]. In these studies, significant differences between the groups in term of fatigue, insomnia, hot flushes, and sexual impairment have been reported, but overall QoL remained unchanged between the study groups. However, the role of fatigue on QoL was not addressed in depths with appropriate instruments. Further, these studies acknowledge that acceptance of morbidity is closely linked to the chance of cure in these patients and significant impairment of function and bother has to be discussed in an elderly population in whom age and organ toxicity is associated with radiotherapy [Citation26,Citation30].
Our findings add to the body of evidence described in previous studies on the significance of QoL for fatigue in PCa survivors [Citation8,Citation9].
Strengths and limitations
Our study has some limitations. It is uncertain who is participating in longitudinal questionnaire studies, thereby, one cannot rule out a selection bias of participants. Adding to this limitation the sample size of the cohort is small and can give rise to statistical type II errors not identifying really significant differences. A further issue is none are PCa-specific, as most patients are elderly men and a decreasing QoL and increasing fatigue with increasing age is well described [Citation26]. Another limitation concerns our national data on fatigue as they were collected in 1996, and concerned men aged 60 years and above [Citation15]. The time differences in data collection and the lack of exact age match should be taken into account when considering the comparative findings.
One strength of our study is the group comparison to a RAD cohort and the uniform inclusion criteria and homogenous treatment applications and the duration of endocrine manipulation in all patients. The use of a validated instrument for the assessment of function and bother, and fatigue on the impairment of QoL is an additional advantage. The impact of oncological outcomes on patient self-rating of QoL will be done in a planned future study. Finally, our attrition analyses at baseline and comparison with normative data should be considered as a strength.
In summary, QoL presumably related to long-term ADT persisted up to 36 months observation and is related both to total fatigue and chronic fatigue.
Conclusions
The significance of cautious patient counseling at baseline to prepare for long-term distress, fatigue, and reduced QoL, as well significantly more bowel and sexual bother over time, has to be implemented when considering curative-intended irradiation with long-term ADT for men with locally advanced PCa. Strategies to improve fatigue in long-term survivors should be addressed and evaluated at the follow-up.
Acknowledgments
The generous support of the study by a grant from the Olav Raagholt and Gerd Meidel Foundation is highly appreciated. We also thank study nurse Melanie-Birte Schulz for her dedication to coordinate follow-up and patient’s care.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Morikawa LK, Roach MIII. Pelvic nodal radiotherapy in patients with unfavorable intermediate and high-risk prostate cancer: evidence, rationale, and future directions. Int. J Radiat Oncol Biol Phys 2011;80:6–16.
- Pinkawa M, Piroth MD, Holy R, Djukic V, Klotz J, Krenkel B, et al. Combination of dose escalation with technological advances (intensity-modulated and image-guided radiotherapy) is not associated with increased morbidity for patients with prostate cancer. Strahlenther Onkol 2011;187:479–84.
- Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:1124–9.
- Lilleby W, Stensvold A, Dahl AA. Adding intensity-modulated radiotherapy to the pelvis does not worsen the adverse effect profiles compared to limited field radiotherapy in men with prostate cancer at 12-month follow-up. Acta Oncol 2014;53:1380–9.
- Zelefsky MJ, Yamada Y, Fuks Z, Zhang Z, Hunt M, Cahlon O, et al. Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys 2008;71:1028–33.
- Lips I, Dehnad H, Kruger AB, van MJ, van der HU, Battermann J. Health-related quality of life in patients with locally advanced prostate cancer after 76 Gy intensity-modulated radiotherapy vs. 70 Gy conformal radiotherapy in a prospective and longitudinal study. Int J Radiat Oncol Biol Phys 2007;69:656–61.
- Marchand V, Bourdin S, Charbonnel C, Rio E, Munos C, Campion L. No impairment of quality of life 18 months after high-dose intensity-modulated radiotherapy for localized prostate cancer: a prospective study. Int J Radiat Oncol Biol Phys 2010;77:1053–9.
- Kyrdalen AE, Dahl AA, Hernes E, Hem E, Fossa SD. Fatigue in prostate cancer survivors treated with definitive radiotherapy and LHRH analogs. Prostate 2010;70:1480–9.
- Kyrdalen AE, Dahl AA, Hernes E, Cvancarova M, Fossa SD. Fatigue in hormone-naïve prostate cancer patients treated with radical prostatectomy or definitive radiotherapy.. Prostate Cancer Prostatic Dis 2010;13:144–50.
- Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer 2002;94:2511–6.
- Roach MIII, Weinberg V, Nash M, Sandler HM, Mclaughlin PW, Kattan MW. Defining high risk prostate cancer with risk groups and nomograms: implications for designing clinical trials. J Urol 2006;176:S16–20.
- D'amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol 2003;21:2163–72.
- Berg A, Lilleby W, Bruland OS, Fossa SD. 10-year survival and quality of life in patients with high-risk pN0 prostate cancer following definitive radiotherapy. Int J Radiat Oncol Biol Phys 2007;69:1074–83.
- Lilleby W, Stensvold A, Dahl AA. Intensity-modulated radiotherapy to the pelvis and androgen deprivation in men with locally advanced prostate cancer: A study of adverse effects and their relation to quality of life. Prostate 2013;73:1038–47.
- Loge JH, Ekeberg O, Kaasa S. Fatigue in the general Norwegian population: normative data and associations. J Psychosom Res 1998;45:53–65.
- Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care 1998;36:1002–12.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69–77.
- Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D. Development of a fatigue scale. J Psychosom Res 1993;37:147–53.
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998;51:1171–8.
- Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Leach GE, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA 1995;273:129–35.
- Bolla M, de Reijke TM, Van TG, Van den Bergh AC, Oddens J, Poortmans PM, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 2009;360:2516–27.
- Denham JW, Joseph D, Lamb DS, Spry NA, Duchesne G, Matthews J, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): an open-label, randomised, phase 3 factorial trial. Lancet Oncol 2014;15:1076–89.
- Fransson P, Lund JA, Damber JE, Klepp O, Wiklund F, Fossa S, et al. Quality of life in patients with locally advanced prostate cancer given endocrine treatment with or without radiotherapy: 4-year follow-up of SPCG-7/SFUO-3, an open-label, randomised, phase III trial. Lancet Oncol 2009;10:370–80.
- Geinitz H, Thamm R, Scholz C, Heinrich C, Prause N, Kerndl S, et al. Longitudinal analysis of quality of life in patients receiving conformal radiation therapy for prostate cancer. Strahlenther Onkol 2010;186:46–52.
- Pirl WF, Greer JA, Goode M, Smith MR. Prospective study of depression and fatigue in men with advanced prostate cancer receiving hormone therapy. Psychooncology 2008;17:148–53.
- Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250–61.
- Lips IM, Dehnad H, van Gils CH, Boeken Kruger AE, van der Heide UA, van Vaupel M. High-dose intensity-modulated radiotherapy for prostate cancer using daily fiducial marker-based position verification: acute and late toxicity in 331 patients. Radiat Oncol 2008;3:3–15.
- Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet 2011;378:2104–11.
- Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009;373:301–8.
- Denham JW, Steigler A, Joseph D, Lamb DS, Spry NA, Duchesne G, et al. Radiation dose escalation or longer androgen suppression for locally advanced prostate cancer? Data from the TROG 03.04 RADAR trial. Radiother Oncol 2015;115:301–7.

