Abstract
This is a descriptive study of tendon pathology with different structural appearances of repair tissue correlated to immunolocalization of cartilage oligomeric matrix protein (COMP) and type I and III collagens and expression of COMP mRNA. The material consists of nine tendons from seven horses (5–25 years old; mean age of 10 years) with clinical tendinopathy and three normal tendons from horses (3, 3, and 13 years old) euthanized for non-orthopedic reasons.
The injured tendons displayed different repair-tissue appearances with organized and disorganized fibroblastic regions as well as areas of necrosis. The normal tendons presented distinct immunoreactivity for COMP and expression of COMP mRNA and type I collagen in the normal aligned fiber structures, but no immunolabeling of type III collagen. However, immunoreactivity for type III collagen was present in the endotenon surrounding the fiber bundles, where no expression of COMP could be seen. Immunostaining for type I and III collagens was present in all of the pathologic regions indicating repair tissue. Interestingly, the granulation tissues showed immunostaining for COMP and expression of COMP mRNA, indicating a role for COMP in repair and remodeling of the tendon after fiber degeneration and rupture.
The present results suggest that not only type III collagen but also COMP is involved in the repair and remodeling processes of the tendon.
Introduction
Injuries to tendons are a common cause of lameness in the athletic horse as well as in man [Citation1–3]. Healing and rehabilitation after equine tendon injuries are time consuming, and reinjury is common. When comparing different treatment regimes of horses suffering from superficial digital flexor tendon (SDFT) injury, the recurrence rate was around 40% regardless of treatment type [Citation4]. The pathology and matrix composition of injured equine tendons from clinical cases are not well documented. This is the first study to present morphological data on clinically injured equine tendons.
Tendons are composed of a small proportion of cells (tenocytes) that synthesize and support the maintenance of the extracellular matrix (ECM). The fibril-forming type I collagen is the major component responsible for the tensile strength of the ECM. The collagen fibrils, arranged along the tensional axis of the tendon, are fused together to larger units, that is, fibers and fiber bundles (fascicles) [Citation5]. The fascicles are separated by a loose connective tissue including vessels and nerves (the endotenon). Between and within the type I collagen fibers are other important matrix molecules such as type III and V collagens, proteoglycans, and cartilage oligomeric matrix protein (COMP) [Citation6]. Type III collagen that forms thinner fibrils than type I collagen is present in normal tendons, particularly in the endotenon. Type III collagen is also incorporated in the collagen type I fibrils during development where it is involved in the regulation of the fibrillogenesis, preventing the lateral growth of type I collagen [Citation5,7–9].
The proteoglycans, that is, decorin, biglycan, versican, aggrecan, fibromodulin, and lumican, are anionic molecules with different functions in the matrix [Citation5,10,11]. Decorin, biglycan, and fibromodulin have collagen-binding properties and interact with the collagen fibers and other matrix molecules, regulating the ECM assembly, including fibrillogenesis [Citation12,13].
COMP, first identified in cartilage, is an abundant glycoprotein particularly present in the tendon exposed to compressive load [Citation14,15]. COMP that belongs to the thrombospondin gene family is a five-armed molecule [Citation16] consisting of five identical subunits (pentamers) that are joined together in a coiled-coil domain in the N-terminal [Citation17]. Each arm has a collagen-binding domain with the ability to bind to type I, II, and IX collagen molecules [Citation18,19] as well as fibronectin [Citation15]. COMP acts as a catalyst in collagen fibrillogenesis in vitro [Citation20], and there are also indications that COMP acts as a regulator in vivo in the extracellular fibril assembly. Previous studies have shown that the COMP level in the equine tendon increases with maturation up to 3 years of age and then levels off and slowly declines [Citation21,22]. In addition, there are studies indicating that physical activity leads to increased COMP levels in the equine tendon [Citation21] as well as a tendon-specific response to exercise [Citation23]. In addition, high levels of COMP have been found in synovial fluid from digital flexor tendon sheaths of horses with tendon pathology [Citation24], indicating release of fragments from tendon matrix. It has been suggested that these fragments may be candidates of biomarkers for tendon injuries in clinical cases [Citation24].
The etiopathogenesis of tendon injury in horse and man is multifactorial, but, in most cases, a preceding degeneration of the ECM of the tendon is present [Citation3,25–27]. This degeneration impairs the tendon strength and elasticity, which may lead to a structure rupture during normal loading. The matrix degeneration is thought to be a probable normal aging process, which can be accelerated by certain extrinsic factors, such as exercise-induced hyperthermia or hypoxia within the tendon [Citation28,29]. Changes in the macromolecular composition of the tendon ECM has been described in conjunction with aging and high level of physical activity [Citation3,22,29–34]. Type III collagen is synthesized early in the repair process of experimentally injured equine tendons [Citation35] and is suggested to restore part of the physical tensile strength. Later type III collagen fibrils are replaced by more force-resistant type I collagen fibrils with enhanced tensile properties [Citation36,37]. In tendon with collagenase-induced injuries, the expression of COMP (real-time polymerase chain reaction) is increased in tissue treated with adipose-derived nucleated cells compared to untreated [Citation38]. Hence, the localization of COMP and its association with type III and I collagens in the clinically injured tendon is of interest.
Several experimental studies with collagenase-induced tendon injuries have been performed to investigate the healing and repair processes [Citation39,40]. A different relationship between type I and III collagens was found [Citation39], and studies have demonstrated that there are morphological changes, with an increase of type III collagen, in the tendon tissue surrounding the ruptured Achilles tendon in man [Citation41,42]. Similarly, Dahlgren et al. [Citation43] found an increase of type III collagen in collagenase-treated flexor tendons from horses. Increased understanding of the healing and repair processes in the equine tendon is necessary to aid in the development of better treatment strategies.
The localization of COMP within the injured tissue of the digital flexor tendon has not been delineated. In this study, the morphology of the tendon in the normal and repair tissue in equine SDFT is compared. These lesions are presented as clinical cases with differences in duration and severity, hence with different structural appearances. In addition, the morphological changes are correlated to the immunolocalization of key matrix molecules, that is, type I and III collagens and COMP and the expression of COMP mRNA. The hypothesis tested is that COMP and type III collagen are present in the repair tissue.
Material and Methods
This study was approved by the local ethical committee. Nine SDFTs (forelimbs) from seven horses were sampled. The breed, age, and duration of tendon injuries are presented in . The horses were 5–25 years old, with a mean age of 10 years, and those included in this study were high-performance (riding, trotting, and galloping) competing horses with a history of SDFT problems, resulting in euthanasia. The tendon injuries were localized by ultrasound scanning. The injured areas (approximately 2 × 1 cm) with surrounding tissue were presented in the mid-metacarpal region and collected immediately after death. The duration of tendon injury varied, but most of the horses had a disease history of >3 months, except one tendon (A) where the duration was only 3 weeks. The control tendons were collected from three high-performing horses (age: 3, 3, 13 years old) sent to necropsy for non-orthopedic reasons without any history of lameness. Five-millimeter-thin longitudinal slices of the mid-metacarpal areas of the normal and injured tendons were obtained. The specimens were fixed in 4% phosphate-buffered formaldehyde solution, pH 7.4, at room temperature (RT) for 24 hr. The samples were trimmed, dehydrated, embedded in paraffin, cut to 4-μm-thick sections (longitudinally), and stained with Hematoxylin & Eosin (H&E) stain for light microscopy. Additional sections were cut for immunohistochemistry and in situ hybridization.
Table 1. The regions of different structural appearances in the individual tendons, displayed as percentage of total section area
Table 2. The labeling of COMP and type I and III collagens is displayed in the different regions of the tendon in each individual, as immunopositive staining, graded as 0, 1, 2, and 3, where 0 represents no staining and 3 represents a marked staining
Histology
Six different structural categories, including two normal areas and four different types of pathologic tissue regions (described in the result section), were identified. The microscopic appearance of sections from each specimen stained with H&E stain was assessed for the presence of these different structural categories.
Immunohistochemistry
All specimens were sectioned for immunolocalization of COMP and type I and III collagens. The immunostaining procedure is described below.
Cartilage oligomeric matrix protein
After 5-min blocking in 3% H2O2, the sections were washed in phosphate-buffered saline (PBS, 0.01 M, pH 7.4) and incubated with normal goat serum (1:50) for 30 min at RT. The sections were then dried and incubated overnight with a rabbit polyclonal antiserum against bovine COMP (1:1000) [Citation14,22]. Visualization of the antibody–antigen complex was done after the sections were rinsed in PBS, an excess of the second goat anti-rabbit antibody (1:200) was applied for 30 min, and the sections were incubated with the avidin–biotin complex (ABC) (Vector Lab, CA, USA) for 30 min and the color developer 3, 3-diaminobenzidine (DAB).
Type I and III collagens
After 5-min blocking in 3% H2O2, the sections were washed in Tris buffer, pH 7.4, and incubated with normal goat serum (1:50) for 30 min at RT (type I collagen). The sections were dried and incubated with a rabbit polyclonal antiserum against human type I collagen (1:200) (BioGenex, San Ramon, CA, USA) or a monoclonal antibody against human type III collagen (1:1) (BioGenex). The antiserum against type III collagen was tested, in a previous study [22], on normal equine liver and found specific for this species. After rinsing, the second antibody (SS Multilink (1:1), BioGenex) was applied onto the section for 30 min at RT, and finally, the antibody–antigen complex was visualized by the Super Sensitive Detective System (BioGenex) applied for 30 min and the color developer DAB for 3 min.
As controls, nonimmune rabbit serum for COMP and type I collagen, nonimmune mouse serum for type III collagen, and buffer were used instead of the primary antibodies.
The intensity of the immunoreaction was graded, subjectively by two observers, as none, mild, moderate, or intense (0, 1, 2, and 3) in each type of tissue structure.
In Situ Hybridization
For in situ hybridization of COMP mRNA, a cocktail of three 32- to 35-mer oligonucleotide probes complementary to equine COMP mRNA with the following 5′ to 3′ sequences was used; 32-mer: CAG TTA TGC TGC CCG GTC TCA CAC TCG TCA AT, 35-mer: TGT CCT GAG TTG GGT ACC GTC ACG CAG TTA TCC TT, 33-mer: GTC ACA GAC ATC CCC TAT ACC ATC GCC ATC ACT. A mixture of two sense probes (a 33-mer and a 35-mer) was used as a negative control (Cybergene AB, Huddinge, Sweden). The probes were labeled at their 3′ end with digoxigenin-11-deoxyuridine triphosphate (dUTP) by using the DIG Oligonucleotide Tailing Kit (Roche, Mannheim, Germany). Sections from the paraffin-embedded material of the central part of the SDFTs of all horses were dewaxed in xylene for 30 min at 60°C and rehydrated in descending concentration of ethanol and finally rinsed in PBS. The sections were then treated with Proteinase K (5 μg/ml; Roche, Mannheim, Germany) for 30 min at 37°C, rinsed in PBS, and post-fixed in 4% paraformaldehyde for 5 min at 4°C. Thereafter, the sections were acetylated with 0.25% acetic anhydride (Sigma-Aldrich, Stockholm, Sweden) in 0.1 M triethanolamine buffer, pH 7.4, (Sigma), 2 × 5 min.
The sections were first prehybridized in a commercial hybridization solution containing 60% formamide and 5X saline sodium citrate (SSC) buffer (Dako, Glostrup, Denmark) without probe for 2 hr at 37°C. After rinsing the slides in 2X SSC, the sections were overlaid with hybridization solution containing 5 pmol/ml of each anti-sense probe or control sense-probe and incubated overnight in a humidified chamber at 39°C. The slides were first immersed in 2X SSC to remove the cover slips and then washed at 39°C as follows: 2 × 15 min in 2X SSC, in 1X SSC, and finally in 0.25X SSC.
Immunological detection of the bound probe was performed as described in the non-radioactive in situ hybridization application manual by Boehringer Mannheim with slight modifications. Briefly, the sections were blocked for 30 min in tris-buffered saline (TBS) buffer (100 mM Tris-HCl, 150 mM NaCl, pH 7.5) containing 0.1% Tween-20 (Merck, Hohenbrunn, Germany) and 2% normal sheep serum (National Veterinary Institute, Uppsala, Sweden). Subsequently, the sections were overlaid with alkaline phosphatase-conjugated anti-digoxigenin antibody (Roche, Mannheim, Germany) diluted 1:1000 in TBS containing 1% normal sheep serum and incubated for 2 hr at RT. For color development, sections were incubated for 18 hr in the dark at RT with nitro-blue tetrazolium/5-bromo-4-chloro-3′-indolyphosphate (NBT/BCIP) (Roche) substrate solution containing 1 mM levamisole.
The number of cells with positive intracellular staining was evaluated, subjectively by two observers in the different structures, in each section, and graded as 0, 1, 2, and 3, with 0 representing no positive cell and 3 representing a majority of positive cells.
Results
Histology
The two normal areas are composed of aligned fibers and endotenon. Normally aligned fibers are composed of longitudinal bundles with aligned, strictly organized tenocytes (). Endotenon was identified as a thin loose connective tissue, containing vessels, and nerves, separating the fiber fascicles from each other (). The four different types of pathologic tissue regions comprise fibroblastic organized and disorganized as well as necrosis and hemorrhages. Organized fibroblastic regions were characterized by high cellularity with longitudinal fiber bundles and fibroblastic cells, largely oriented longitudinally, but not aligned (). In addition, vessels were present between fibers with more extensive endotenon than in the normal tendon. Granulation tissue classified as disorganized fibroblastic was composed of randomly oriented fiber bundles with large numbers of fibroblastic cells and marked neovascularization (). Regions of necrosis were defined when aligned fiber bundles contained few cells mostly with pyknotic nuclei, and the fiber bundles were disintegrated with a homogenous appearance (). Hemorrhage, seen as extravascular accumulation of erythrocytes (), was found in only one tendon (A). The regions of the different structural classifications were estimated, by two observers, using a morphometric grid (10 × 10) and presented as a percentage of the total section area, which was approximately 2 cm × 3 cm.
Figure 1. Microscopic longitudinal section (H&E stained) of a normal tendon (L). Note the aligned fiber bundles with tenocytes in parallel rows and thin endotenon (arrows). L refers to tendon ID (see Tables 1 and 2). Bar = 100 μm.
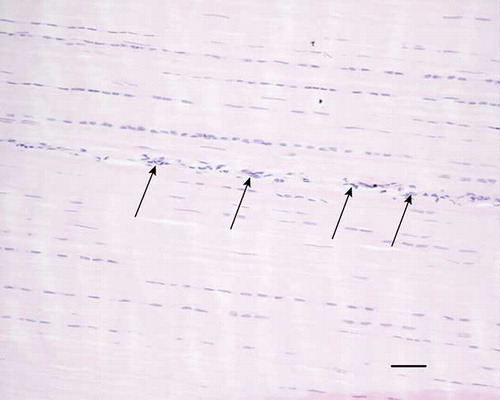
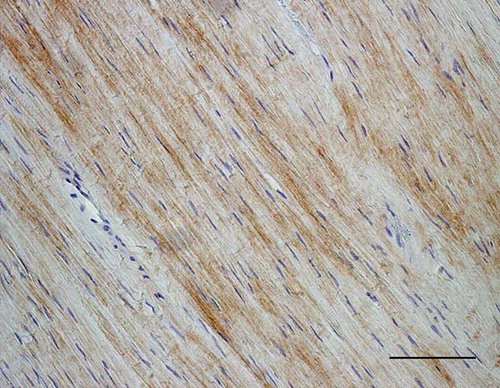
Figure 2. Microscopic longitudinal section (H&E stained) of an injured tendon (C) displaying an organized fibroblastic region. There is a high cellularity with fibroblastic cells and fibers in longitudinal parallel arrangement. Vessels are present between fibers (arrows). C refers to tendon ID (see Tables 1 and 2). Bar = 200 μm.
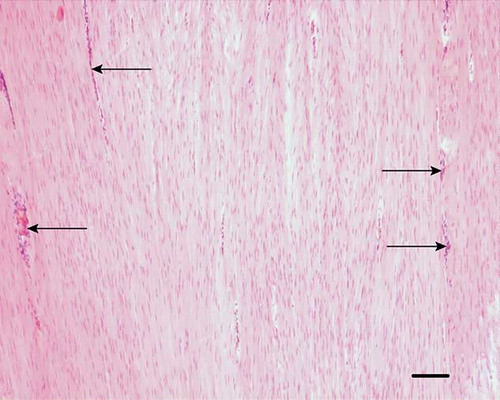
Figure 3. Microscopic longitudinal section (H&E stained) of an injured tendon (H) showing a disorganized region with high amount of fibroblastic cells (arrows) and many vessel-like structures (arrowheads) compatible with neovascularization. H refers to tendon ID (see Tables 1 and 2). Bar = 100 μm.
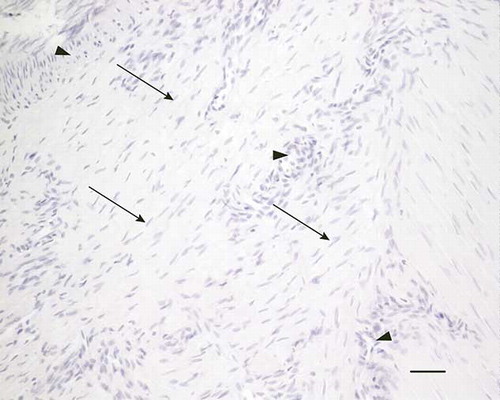
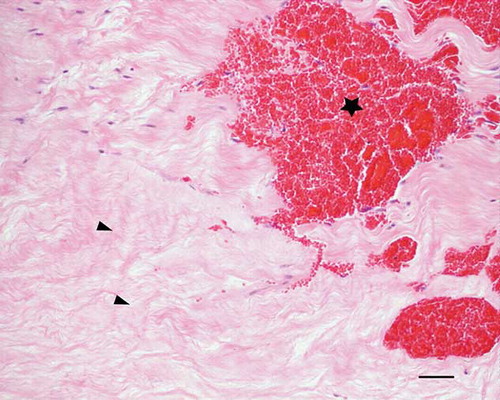
Figure 4. Microscopic longitudinal section (H&E stained) of injured tendon (A) characterized by acellular necrotic area (arrows) and hemorrhages (*). A refers to tendon ID (see Tables 1 and 2). Bar = 100 μm.
The distribution in percentage of the total region of the different structural appearances is presented in . The normal tendons displayed aligned fiber bundles separated by a thin endotenon () without any granulation/repair tissue structures.
One of the injured tendons (A) contained regions of hemorrhage, suggesting an acute damage, together with large areas (45%) of necrosis (). This tendon was obtained from a horse with a more acute clinical tendinopathy (3 weeks). All of the injured tendons demonstrated organized fibroblastic regions () ranging from 5 to 80% of the total section compared to normal tendons where no such regions were found (). All but one (F) of the injured tendons showed regions of disorganized fibroblastic tissue (). All tendons contained endotenon (), except one (B). The major part of the section from tendon B (95%) was characterized by a mixture of fibroblastic organized (30%) and disorganized (60%) regions and necrotic areas (5%), without recognizable normal endotenon. This disorganized granulation tissue was characterized by marked vascularization and predominant fibroblastic cells without identifiable fiber bundles.
Immunohistochemistry and In Situ Hybridization
The immunolabeling of COMP and type I and III collagens and in situ expression of COMP are displayed in . No staining was seen when the primary specific antibody was omitted or replaced with normal control serum in the incubation. No positive cells could be detected with the control sense probe.
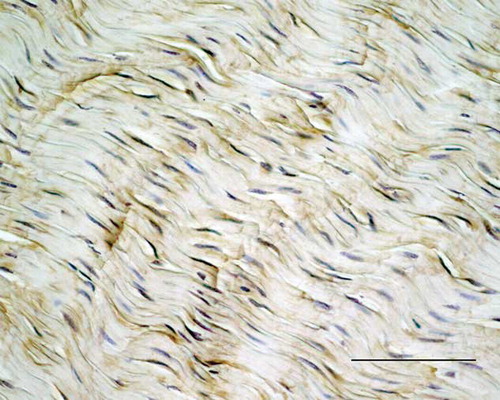
COMP staining was present to some extent in all sections, with the most intense staining in the normally aligned fiber structures (), where expression of COMP mRNA could also be seen in the cytoplasm of the majority of the cells. The endotenon mostly lacked immunoreactivity for COMP and lacked expression of COMP mRNA in a majority of the tendons. The exception being A, E, F, and G, where COMP staining and expression of COMP mRNA could be seen in part of the endotenon. In two of these tendons (A and E), large areas of necrosis were found. There was no immunostaining for COMP in the endotenon of normal tendons (controls), and these showed no cells positive for expression of COMP mRNA.
Figure 5. Microscopic longitudinal section of a normal tendon (K). Immunohistochemical localization of COMP. Note the staining in the normally aligned fibers. K refers to tendon ID (see Tables 1 and 2). Bar = 100 μm.
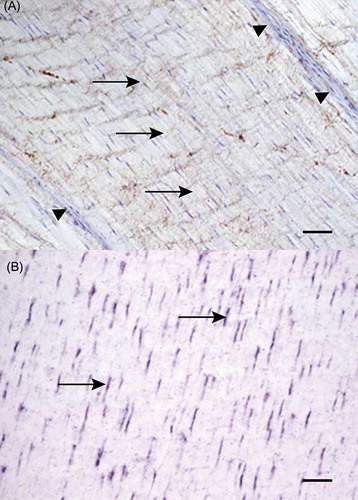
Interestingly, the fibroblastic organized or disorganized regions showed mild-to-moderate staining for COMP in most of the tendons as well as cells expressing COMP mRNA ().
Figure 6. Microscopic longitudinal section of an injured tendon (C). A: Immunohistochemical localization of COMP in a fibroblastic organized region with a marked staining (arrows) and in endotenon (arrowheads). Bar = 100 μm. B: In situ hybridization with the expression of COMP m-RNA (dark stain) in the cytoplasm of the majority of cells (arrows) in the same region as a). C refers to tendon ID (see Tables 1 and 2). Bar = 100 μm.
Antibodies against type I collagen gave a clear staining pattern in all normally aligned fiber structures. In contrast, the endotenon displayed no or weak staining. Most of the fibroblastic regions, both such organized and disorganized, showed intense immunolabeling for type I collagen (). Also the areas of necrosis displayed intense immunoreactivity for type I collagen.
Figure 7. Microscopic longitudinal section of injured tendon (H). Immunohistochemical localization of type I collagen. Note the intense staining of the organized fibroblastic tissue, but lack of staining in the extensive endotenon (arrows). H refers to tendon ID (see Tables 1 and 2). Bar = 100 μm.
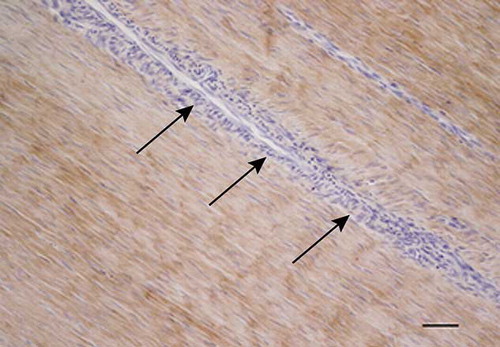
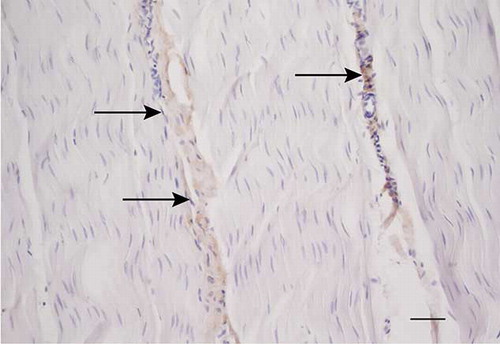
Antibodies against type III collagen gave some staining in all tendons. However, in the normal tendons, the immunolabeling was restricted to the endotenon areas (). This was not true for the injured tendons, where staining could be seen in the different types of granulation/repair tissue and in the endotenon. In some of the injured tendons, the most intense immunoreactivity for type III collagen was observed in the fibroblastic, disorganized regions () and the necrotic areas as well as in the small areas of aligned fibers.
Figure 8. Microscopic longitudinal section of a normal tendon from a control (K). Immunohistochemical localization of type III collagen. A marked immunostaining is present in the endotenon (arrows), but the aligned fibers do not show any staining. K refers to tendon ID (see Tables 1 and 2). Bar = 100 μm.
Discussion
The aim of this study was to characterize the repair tissue in clinically injured equine SDFTs, with reference to type I and III collagens and COMP.
Type III collagen immunostaining was found in all the fibroblastic organized and disorganized regions. These regions represent repair tissue of different age/maturation. This is in accordance with previous investigations showing that type III collagen synthesis is increased 1 week after collagenase induced injury of the equine SDFTs [Citation43] as well as an increased presence in ovine tendon [Citation44]. In tendons from horses without any history of clinical tendon problems, however, with presence of macroscopic “degeneration” in the central core of the SDFTs, an increase in type III collagen levels has been reported [Citation31]. Also, in material from spontaneously ruptured human Achilles tendons, showing microscopic fiber degeneration and tenocyte necrosis, an increased amount of type III collagen was present in the tissue of aligned collagen fibers surrounding the rupture [Citation26,41,45]. These findings suggest clinically silent degeneration of collagen fibers, predisposing to tendon rupture even at normal mechanical load, both in man and in horse [Citation26,31]. The type III collagen immunostaining seen in the minor areas of morphologically normally aligned fiber structures described in four of the injured tendons in this study may indicate early fiber degeneration, predisposing to fiber rupture.
As expected, intense immunostaining for type I collagen was present in the aligned fibers, in fibroblastic organized and disorganized granulation tissues, and in areas of necrosis in all of the tendons. This intense staining for type I collagen was seen in both injured and normal tendons, expected in view of the ubiquitous presence of this collagen type. A minor immunoreactivity for type I collagen was found in the endotenon, characterized by a loose connective tissue including blood and lymphatic vessels and nerves.
Immunoreactivity for COMP appeared in a similar pattern as reactivity for type I collagen in the normal tendons, with marked staining in the aligned fibers and almost no staining in the endotenon. In addition, the presence of COMP mRNA was observed in many cells of the normally aligned fiber structures and absent in the cells of the endotenon. This has also been reported in an earlier study of normal SDFTs from adult horses [Citation22].
COMP has been found in skin wound tissue [Citation46] and in serum of scleroderma patients [Citation47], suggested as a marker for fibroblast activation. It is also reported to be over-expressed by scleroderma dermal fibroblasts and suggested to play a role in new repair matrix deposition [Citation46]. This upregulation of COMP has been suggested to be affected by transforming growth factor-β (TGF-β). However, there was no correlation between TGF-β1 and COMP concentrations during wound healing in equine skin [Citation48].
The granulation tissues including necrotic areas of the injured tendons in this study, displayed immunoreactivity for COMP with mild-to-moderate staining in most of the tendons examined. In addition, COMP mRNA was observed in most of the cells (grade 2–3) of the organized fibroblastic regions and in some of the cells (grade 1–3) in the disorganized fibroblastic regions. In one of the tendons (B), with a large amount of fibroblastic, disorganized regions, the immunostaining for COMP was not apparent. However, in situ hybridization indicated a moderate number of cells with expression of COMP in the disorganized regions. In this specimen, the fibroblastic, disorganized region had an atypical appearance with marked vascularization, high cellularity by fibroblastic cells without fiber bundles, and no identifiable endotenon. The absence of immunostaining for COMP, but presence of COMP mRNA in the cytoplasm of cells in these regions, may indicate early expression or high protein turnover. This granulation tissue may contain many different proteolytic enzymes involved in the remodeling of a disorganized tissue, where the large number of cells surrounded by few collagen fibers may lead to loss of COMP.
The repair tissue of these clinically injured equine tendons clearly showed the presence of type III collagen, cells producing COMP, and COMP in the ECM. This strengthens the suggestions that COMP present in the synovial fluid of tendon sheaths (Smith et al. [Citation24]) is released from fragmented tendons and that COMP and/or COMP fragments in the synovial fluid might be used as a biomarker-monitoring tendon in the tendon repair process.
Conclusion
In conclusion, the immunolocalization of type I and III collagens in injured equine tendons is in accordance with previous studies on experimental injured tendons from horse and ruptured tendons from man. The present results suggest, for the first time in equine clinical material, that type III collagen and COMP are involved in the repair and remodeling processes of the tendon. COMP may serve as a biomarker for tendon healing and repair processes. Further research into COMP synthesis, structural assembly, and localization in the ECM is warranted. Research into the biological turnover in the normal and injured tendon of equine athletes would aid in the understanding of the etiopathogenesis and hence in the prevention of exercise-induced tendinopathy.
Acknowledgments
We thank technical assistants Åsa Gessbo and Agneta Boström for excellent help with sectioning, immunohistochemistry- and in situ hybridization.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the article.
This study was supported by grants from FORMAS (The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning), ATG (The Research committee of the Swedish Horserace Totalizator Board), and Professor Gerhard Forssells stipendiestiftelse.
References
- Williams, R.B., Harkins, L.S., Hammond, C.J., and Wood, J.L. (2001). Racehorse injuries, clinical problems and fatalities recorded on British racecourses from flat racing and National Hunt racing during 1996, 1997 and 1998. Equine Vet. J. 33:478–486.
- Nyyssonen, T., and Luthje, P. (2000). Achilles tendon ruptures in South-East Finland between 1986–1996, with special reference to epidemiology, complications of surgery and hospital costs. Ann. Chir. Gynaecol. 89:53–57.
- Thorpe, C.T., Clegg, P.D., and Birch, H.L. (2010). A review of tendon injury: Why is the equine superficial digital flexor tendon most at risk? Equine Vet. J. 42:174–180.
- Dyson, S.J. (2004). Medical management of superficial digital flexor tendonitis: A comparative study in 219 horses (1992–2000). Equine Vet. J. 36:415–419.
- Benjamin, M., and Ralphs, J.R. (1997). Tendon and ligaments—An overview. Histol. Histopathol. 12:1135–1144.
- Lapiere, C.M., Nusgens, B., and Pierard, G.E. (1977). Interaction between collagen type I and type III in conditioning bundles organization. Connective Tissue Res. 5:21–29.
- Wang, J.H.-C. (2006). Mechanobiology of tendon—Review. J. Biomechanics. 39:1563–1582.
- Zhang, G., Young, B.B., Ezura, Y., Favata, M., Soslowsky, L.J., Chakravarti, S., and Birk, D.E. (2005). Development of tendon structure and function: Regulation of collagen fibrillogenesis. J. Musculoskelet. Neuronal. Interact. 5:5–21.
- Birk, D.E., and Mayne, R. (1997). Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol. 72:352–361.
- Abrahamsson, S.O. (1991). Matrix metabolism and healing in the flexor tendon—Review. Scand. J. Plast. Reconstr. Surg. Handsurg. 23(Suppl.):1–51.
- Vogel, K.G., Sandy, J.D., Pogány, G., and Robbins, J.R. (1994). Aggrecan in bovine tendon. Matrix Biol. 14:171–179.
- Svensson, L., Aszódi, A., Reinholt, F.P., Fässler, R., Heinegård, D., and Oldberg, Å. (1999). Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J. Biol. Chem. 274:9636–9647.
- Graham, H.K., Holmes, D.F., Watson, R.B., and Kadler, K.E. (1999). Identification of Collagen fibril fusion during vertebrate tendon morphogenesis. The process relies on unipolar fibrils and is regulated by collagen-proteoglycan interaction. J. Mol. Biol. 295:891–902.
- Hedbom, E., Antonsson, P., Hjerpe, A., Aeschlimann, D., Paulsson, M., Rosa-Pimentel, E., Sommarin, Y., Wendel, M., Oldberg, Å., and Heinegård, D. (1992). Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J. Biol. Chem. 267:6132–6136.
- Di Cesare, P., Hauser, N., Lehman, D., Pasumarti, S., and Paulsson, M. (1994). Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 354:237–240.
- Mörgelin, M., Heinegård, D., Engel, J., and Electron, P.M. (1992). Microscopy of native cartilage oligomeric matrix protein purified from the swarm rat chondrosarcoma reveals a five-armed structure. J. Biol. Chem. 267:6137–6141.
- Oldberg, A., Antonsson, P., Lindblom, K., and Heinegård, D. (1992). COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J. Biol. Chem. 267:22346–22350.
- Holden, P., Meadows, R.S., Chapman, K.L., Grant, M.E., Kadler, K.E., and Briggs, M.D. (2001). Cartilage oligomeric matrix protein interacts with type IX collagen, and disruptions to these interactions identify a pathogenetic mechanism in a bone dysplasia family. J. Biol. Chem. 276:6046–6055.
- Rosenberg, K., Olsson, H., Mörgelin, M., and Heinegård, D. (1998).Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J. Biol. Chem. 273:20397–20403.
- Halász, K., Kassner, A., Mörgelin, M., and Heinegård, D. (2007).COMP acts as a catalyst in collagen fibrillogenesis. J. Biol. Chem. 282:31166–31173.
- Smith, R.K.W., Zunino, L., Webbon, P.M., and Heinegård, D. (1997). The distribution of cartilage oligomeric matrix protein (COMP) in tendon and its variation with tendon site, age and load. Matrix Biol. 16:255–271.
- Södersten, F., Ekman, S., Eloranta, M.-L., Heinegård, D., Dudhia, J., and Hultenby, K. (2005). Ultrastructural immunolocalization of cartilage oligomeric matris protein (COMP) in relation to collagen fibrils in the equine tendon. Matrix Biol. 24:376–385.
- Kasashima, Y., Takahashi, T., Birch, H.L., Smith, R.K.W., and Goodship, A.E. (2008).Can exercise modulate the maturation of functionally different immature tendons in the horse? J. Appl. Physiol. 104:416–422.
- Smith, M.R.W., Wright, I.M., Minshall, G.J., Verheyen, K., Heinegård, D., and Smith, R.K.W. (2011). Increased cartilage oligomeric matrix protein concentrations in equine digital flexor tendon sheath synovial fluid predicts intrathecal tendon damage. Vet. Surg. 40:54–58.
- Goodship, A.E., Birch, H.L., and Wilson, A.M. (1994). The pathobiology and repair of tendon and ligament injury. Vet Clin. North Am. Equine Pract. 10:323–349.
- Cetti, R., Junge, J., and Vyberg, M. (2003). Spontaneous rupture of the Achilles tendon is preceded by widespread and bilateral tendon damage and ipsilateral inflammation: A clinical and histopathologic study of 60 patients. Acta Orthop. Scand. 74:78–84.
- Riley, G. (2008). Tendinopathy – From basic science to treatment. Rheumatology 4:82–89.
- Wilson, A.M., and Goodship, A.E. (1994). Exercise-induced hyperthermia as a possible mechanism for tendon degeneration. J. Biomechanics. 27:899–905.
- Smith, R.K.W., Birch, H.L., Goodman, S., Heinegård, D., and Goodship, A.E. (2002). The influence of ageing and exercise on tendon growth and degeneration—Hypotheses for the initiation and prevention of strain-induced tendinopathies. Comp. Biochem. Physiol. A Mol. Intergr. Physiol. 133:1039–1050.
- Jones, A.J., and Bee, J.A. (1990). Age- and position-related heterogeneity of equine tendon extracellular matrix composition. Res. Vet. Sci. 48:357–364.
- Birch, H.L., Bailey, A.J., and Goodship, A.E. (1998). Macroscopic ‘degeneration’ of equine superficial digital flexor tendon is accompanied by a change in extracellular matrix composition. Equine Vet. J. 30:534–539.
- Birch, H.L., Bailey, J.V.B., Bailey, A.J., and Goodship, A.E. (1999). Age-related changes to the molecular and cellular components of equine flexor tendons. Equine Vet. J. 31:391–396.
- Cherdchutham, W., Becker, C., Smith, R.K.W., Barneveld, A., and van Weeren, P.R. (1999). Age-related changes and effect of exercise on the molecular composition of immature equine superficial digital flexor tendons. Equine Vet. J. 31(Suppl.):86–94.
- Cherdchutham, W., Becker, C.K., Spek, E.R., Voorhout, W.F., and van Weeren, P.R. (2001). Effects of exercise on the diameter of collagen fibrils in the central core and periphery of the superficial digital flexor tendon in foals. Am. J. Vet. Res. 62:1563–1570.
- Dahlgren, L.A., Brower-Toland, B.D., and Nixon, A.J. (2005). Cloning and expression of type III collagen in normal and injured tendons of horses. Am. J. Vet. Res. 66:266–271.
- Williams, I.F., McCullagh, K.G., and Silver, I.A. (1984). The distribution of types I and III collagen and fibronectin in the healing equine tendon. Connective Tissue Res. 12:211–227.
- Watkins, J.P., Auer, J.A., Gay, S., and Morgan, S.J. (1985). Healing of surgically created defects in the equine superficial digital flexor tendon: Collagen-type transformation and tissue morphologic reorganization. Am. J. Vet. Res. 46:2091–2096.
- Nixon, A.J., Dahlgren, L.A., Haupt, J.L., Yeager, A.E., and Ward, D.L. (2008). Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am. J. Vet. Res. 69:928–937.
- Williams, I.F., McCullagh, K.G., Goodship, A.E., and Silver, I.A. (1984). Studies on the pathogenesis of equine tendonitis following collagenase injury. Res. Vet. Sci. 36:326–338.
- Watts, A.E., Nixon, A.J., Yeager, A.E., and Mohammed, H.O. (2012). A collagenase gel/physical defect model for controlled induction of superficial digital flexor tendonitis. Equine Vet. J. 44:576–386. doi: 10.1111/j.2042-3306.2011.00471.x.
- Eriksen, H.A., Pajala, A., Leppilahti, J., and Risteli, J. (2002). Increased content of type III collagen at the rupture site of human Achilles tendon. J. Orthop. Res. 20:1352–1357.
- Jozsa, L., and Kannus, P. (1997). Histopathological findings in spontaneous tendon ruptures. Scand. J. Med. Sci. Sports. 7:113–118.
- Dahlgren, L.A., Mohammad, H.M., and Nixon, A.J. (2005). Temporal expression of growth factors and matrix molecules in healing tendon lesions. J. Orthop. Res. 23:84–92.
- Crovace, A., Lacitignola, L., Francioso, E., and Rossi, G. (2008). Histology and immunohistochemistry study of ovine tendon grafted with cBMSCs and BMMNCs after collagenase-induced tendinitis. Vet. Comp. Orthop. Traumatol. 21:329–336.
- Maffulli, N., Ewen, S.W., Waterston, S.W., Reaper, J., and Barrass, V. (2000). Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing. Am. J. Sports Med. 28:499–505.
- Farina, G., Lemaire, R., Korn, J.H., and Widom, R.L. (2006). Cartilage oligomeric matrix protein is over expressed by scleroderma dermal fibroblasts. Matrix Biol. 25:213–222.
- Hesselstrand, R., Kassner, A., Heinegård, D., and Saxne, T. (2008). COMP: A candidate molecule in the pathogenesis of systemic sclerosis with a potential as a disease marker. Ann. Rhem. Dis. 67:1242–1248.
- Dart, A.J., Dart, C.M., Dudhia, J., Perkins, N., Canfield, P., and Smith, R.K. (2011). A preliminary study on the effect of wounding on transforming growth factor-β1 and cartilage oligomeric matrix protein concentrations in the skin of horses. Vet. Surg. 40:59–65.