Abstract
Introduction. Left atrial catheter ablation is useful as symptomatic treatment in selected patients with atrial fibrillation (AF). Evaluation requires measurement of arrhythmia-related symptoms. Many of the published protocols have drawbacks and have been used in AF only, with no possible comparison to other ablations that compete for the same resources. U22 is a published protocol that quantifies paroxysmal tachycardia symptoms through scales with 11 answer alternatives, translated into discrete numerical scales 0–10. It has been shown to reflect the clinical improvement after ablation of supraventricular tachycardia. Here we report the use of U22 in measuring improvement after catheter ablation for AF. Material and methods. A total of 105 patients underwent first-time ablation for AF and answered U22 and SF-36 forms at baseline and follow-up 304 (SD 121) days after ablation. Independently, the patients underwent a clinical follow-up. All decisions regarding medication and reablation were taken without knowledge of the symptom scores. Results. The U22 scores for well-being, arrhythmia as cause for impaired well-being, derived time-aspect score for arrhythmia, and discomfort during attack detected relevant improvements of symptoms after the ablation. U22 showed larger improvement in patients undergoing only one procedure than in patients who later underwent repeated interventions, thus reflecting the independent clinical decision for reablation. Conclusion.U22 quantifies the symptomatic improvement after AF ablation with adequate internal consistency and construct validity. U22 mirrors aspects of the arrhythmia symptomatology other than SF-36.
Introduction
Left atrial catheter ablation is a suitable treatment in selected patients with atrial fibrillation. The patients experience improvement due both to decreased incidence of arrhythmia and decreased symptoms from remaining arrhythmias (Citation1). The procedure is demanding and competes for limited resources with other electrophysiological interventions. No prognostic gain has yet been shown, and the indication for the procedure presently is only symptomatic. Tools for measurement of arrhythmia-related symptoms are therefore much needed for evaluation and comparison between different groups. Several questionnaires that quantify symptoms associated with tachyarrhythmias have been published. The Symptom Checklist-Frequency and Severity Scale exists in multiple versions that are not well-described in the literature (Citation2-6). The ASTA-questionnaire (Citation7) is a three-part protocol that measures arrhythmia-specific symptoms and health-related quality of life in connection with arrhythmias. Most of the other protocols have been developed and used only in atrial fibrillation (Citation8-16), thus offering no comparison with the large groups of other arrhythmias that also undergo ablation on symptomatic indication and compete for the same resources. U22 is a patient-administered protocol that quantifies paroxysmal symptoms associated with tachyarrhythmias. The protocol has been published and shown to reflect the clinical improvement after ablation of paroxysmal supraventricular tachycardia, a procedure considered as curative with a distinct end-point and a high success rate (Citation17,18). Our present aim is to quantify the symptomatic effect of left atrial catheter ablation for atrial fibrillation with U22 and relate the results to the incidence of reablation based on the clinical evaluation that serves as reference.
Material and methods
Patients
The study group consists of patients who underwent left atrial catheter ablation for paroxysmal and persistent atrial fibrillation at the Heart Centre, University Hospital, Umeå, Sweden, between 2006 and 2011. On admission, the patients were invited to answer the baseline U22 and SF-36 forms. Radiofrequency ablation was subsequently performed as segmental pulmonary vein isolation, wide antral circumferential isolation with an irrigated tip catheter, and the CARTO® mapping system (Biosense Webster, Inc., Diamond Bar, CA, USA) or isolation with a multi-polar circular ablation catheter (PVAC®, Medtronic, Inc., Minneapolis, MN, USA). A questionnaire follow-up with U22 and SF-36 was performed 6–9 months after the ablation. The answers were prospectively entered into a database by a co-author (T.T.), blinded with respect to the clinical picture. Independently from the U22 protocol, the patients underwent a clinical follow-up (co-authors N.H., F.R., S.M.J., M.K.) and a 7-day Holter-monitor recording. All decisions regarding medication and possible repeated ablation were taken without knowledge of the symptom scores. The protocol data were subsequently retrieved for the analysis. At review time the clinical data and the catheterization reports were analysed and coded according to a pre-specified scheme by co-authors blinded with respect to the U22 results (N.H., F.R., S.M.J., M.K.).
Questionnaires
The arrhythmia-related symptoms were measured with the U22 protocol. Detailed description and the definitions of the individual U22 scores have been published previously (Citation17,18). Symptoms are quantified by positioning the answers on verbally described scales with 11 horizontally aligned answer alternatives, an approach similar to that by Härden et al. (Citation10). The answers are translated into discrete numerical scales with a range of 0–10 (NRS-10, a Likert scale that is a discrete alternative of the common visual analogue scale). In the current analysis the following U22 questions were used:
q01: ‘On the whole, how have you felt over the past month?' [miserable–very well], [0–10]
q02 (follow-up only): ‘Compared to the time before the treatment, do you now feel:' [Very much worse–very much better], [0–10]
q03: ‘Do you take any prescribed medications for your heart rhythm problems?' [No, Yes]
q06 (follow-up only): ‘Have you experienced any problems with the heart rhythm after the treatment? – please disregard the first 3 months after the treatment –' [No; Yes, of the same type as before the treatment; Yes, of different type]
q08: ‘How often do you experience problems with heart rhythm? (despite taking any medication)' [Never, Rarely, A few times a month, A few times a week, Daily, All the time]
q10: ‘How long does a spell usually last?' [Seconds, More than a minute but less than 15 min, 15 min to 1 hour, 1 to 4 hours, More than 4 hours, All the time]
q11: ‘How much do the spells affect your well-being?' [Not at all–very much], [0–10]
q12. ‘How bothered are you while you are experiencing a spell?' [Not at all–very much], [0–10]
A time-aspect with range 0–10 was computed by summarizing the scores from q08 and q10 (Citation18).
SF-36 (Citation19) was used as a generic measure of quality of life. It quantifies the mental and physical well-being in eight scales with range 0–100, together with two summary scores, Physical Component Summary (PCS) score and Mental Component Summary (MCS) score.
Answers to U22 and SF-36 were compared between baseline and follow-up, group-wise and in individual patients.
Statistics
Data are presented as mean (SD), unless otherwise stated. Differences between groups in continuous variables were examined by paired and unpaired two-tailed t test and global F test. Pearson's r was used for correlation between continuous variables. Cronbach's alpha was used for estimating internal consistency of the scores. For analysis of freedom from subsequent reablation, Kaplan–Meier curves were constructed, and differences between dichotomized groups were evaluated by log-rank test. Two-tailed Fisher exact text and chi-square test were used for testing differences in proportions. A p-level of 0.05 was considered as significant. The data were analysed in R 2.15.2 (R Foundation for Statistical Computing, 2012, http://www.R-project.org). The study was approved by the Ethics Committee at the Umeå University Faculty of Medicine.
Results
A total of 105 patients undergoing a first-time left atrial ablation on clinical indication answered all four required forms (U22 and SF-36 at baseline and follow-up). The group consisted of 78 men and 27 women aged 58 (SD 9) years.
Clinical description
The atrial fibrillation was paroxysmal in 50%, persistent in 48%, and long-standing persistent in 2% of the patients. At baseline, the CHADS2 scores were 0, 1, 2, 3, and 4 in 49%, 35%, 11%, 4%, and 1% of patients, respectively. The mean left atrial diameter was 44 (SD 7) mm. Segmental pulmonary vein isolation was performed in 36 cases (34%), wide antral circumferential isolation in 43 cases (41%), and isolation with the multi-polar catheter in 26 cases (25%). The procedure and fluoroscopy times were 208 (SD 50) and 36 (SD 13) minutes.
At the clinical follow-up 136 (SD 58) days after the ablation, 62% of the patients had no atrial fibrillation in the 7-day Holter-monitor recording. In 61% of the patients, the symptomatic result was considered satisfactory, and antiarrhythmic drugs were discontinued. In 20% the result was considered as satisfactory, but the antiarrhythmic therapy was continued. In 14% the result was not satisfactory, and a second catheter ablation was intended. In 5% the result was not satisfactory, but no catheter intervention was planned at the clinical follow-up (due to patient's wish, permanent atrial fibrillation, or planned surgical ablation). The patient records were analysed at review time 873 (SD 490) days after the first-time ablation. At that time the incidence of reablation was 34%, while 66% had only undergone the initial ablation.
U22 in all first-time ablations
At the questionnaire follow-up 304 (SD 121) days after the ablation, 29 patients (28%) reported freedom from arrhythmia symptoms, 48 (46%) had the same type of symptoms as before the ablation, and 27 (26%) had symptoms of different type (q06). Eighty (78%) patients reported taking some medication (including beta-blockers) against the arrhythmia, compared to 96 (91%) prior to the ablation (q03).
The main results are summarized in and . Compared to the state before ablation, significant improvements were recorded at follow-up in the U22 scores for well-being (q01), arrhythmia as cause for impaired well-being (q11), the time-aspect score for arrhythmia derived from q08 and q10 (Citation17,18), discomfort during an attack (q12), and the SF-36 summary scores PCS and MCS.
Table I. Patient profile, U22, and SF-36 in 105 ablated patients.
Table II. Singular and repeated ablations.
No significant differences between the three ablation techniques were seen in improvement of well-being (expressed as difference q01follow-up – q01baseline, F = 1.26, p = 0.3), arrhythmia as cause for impaired well-being (difference q11follow-up – q11baseline, F = 0.17, p > 0.5), or discomfort during an attack (difference q12follow-up – q12baseline, F = 0.12, p > 0.5). No significant differences in these parameters were detected between the groups with CHADS2 = 0, 1, and >1.
The score for patients' retrospective estimation of improvement in well-being measured at follow-up (q02) was 7.1 (SD 2.5). This retrospective estimate of improvement correlated moderately to the improvement computed as difference between the score for well-being at follow-up and before ablation (q01follow-up – q01baseline) (r = 0.55, p < 0.0001).
At baseline, U22 score for well-being (q01) correlated moderately to the SF-36 summary variables PCS and MCS (r = 0.65 and 0.49). The correlations between q11, q12, and time-aspect on one side and PCS and MCS on the other side were weak (r ≤ 0.4) or non-significant.
At follow-up, q01 correlated strongly to PCS and moderately to MCS (r = 0.75 and 0.54); and q11, q12, and time-aspect correlated moderately to PCS (r = 0.59, 0.57, and 0.61) and weakly to MCS (r ≤ 0.4). The strong and moderate correlations were significant (p ≤ 0.0004, corrected for multiple comparisons by Holm's method).
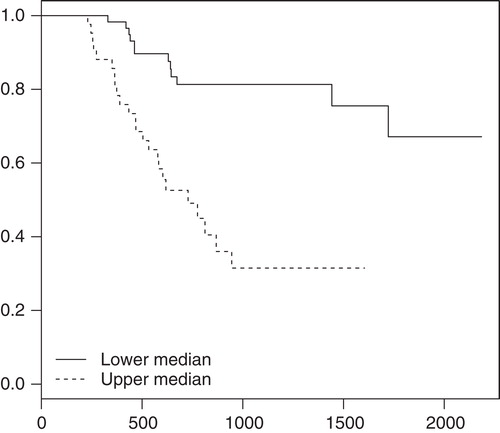
Patients had a low incidence of reablation at review time if their first-time ablation was associated with a high improvement of U22 scores ().
Figure 1. Symptomatic improvement in 105 patients and freedom from subsequent reablation. Survival curves for freedom from reablation as a function of days after first-time ablation. The population was dichotomized into two subsets along the median of individual patients' differences in U22 scores, computed as (scorefollow-up – scorebaseline). The plot is shown for the U22 score q11 (effect of arrhythmia on the well-being), p < 0.0001 for the difference between the survival curves. A similar pattern was seen in the U22 scores q01, q12, and time-aspect of arrhythmia (p = 0.0006, p < 0.0001, and p < 0.0001, respectively).
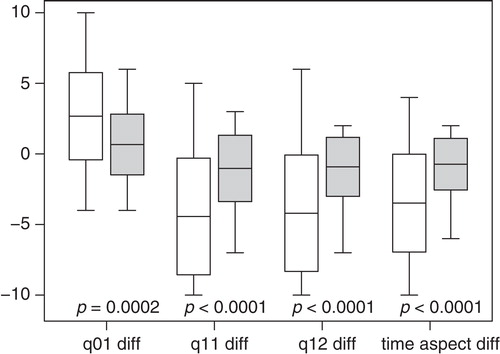
U22 in singular procedure, compared to the first of repeated procedures
Of the above patients 69 underwent only one procedure. They were compared to the 36 patients who later underwent repeated procedures (). At follow-up, U22 detected a significantly larger symptomatic improvement in the scores for well-being (q01), arrhythmia as cause for impaired well-being (q11), discomfort during an attack (q12), and derived time-aspect score for arrhythmia in the patients who underwent a singular ablation than in the group with the first of multiple ablations ().
Figure 2. Differences in U22 scores in singular ablations compared to the first of multiple ablations. The differences for q01, q11, q12, and time-aspect in individual patients were computed as (scorefollow-up – scorebaseline). Singular ablations are represented by white boxes, the first of multiple ablations by grey boxes. The boxes are delimited by mean ± 1 SD. The central line depicts the mean, and the whiskers are placed at the extreme values. For all scores the singular ablations resulted in significantly larger improvements than the first of multiple ablations.
Most recent of repeated procedures
Nineteen patients who had undergone repeated procedures (mean 2.11/patient) answered all four forms in connection with their most recent procedure. After this last ablation, the U22 scores and the SF-36 summary scores had improved to a similar degree as after a singular ablation (). Their improvement in the U22 scores and the SF-36 summary scores did not significantly differ from that of the 75 patients undergoing a singular procedure.
Freedom from atrial fibrillation in the follow-up Holter recording
The patients with freedom from atrial fibrillation in the 7-day Holter-monitor recording at clinical follow-up had significantly better mean U22 scores for well-being (q01), arrhythmia as cause for impaired well-being (q11), the time-aspect score for arrhythmia, and discomfort during an attack (q12) compared to those with some episode of atrial fibrillation (6.9 versus 4.4; 3.9 versus 6.7; 3.3 versus 5.7; and 4.1 versus 6.8, respectively, p < 0.0005 for all comparisons). At review time 27% of the former had undergone reablation, compared to 67% of the latter (log-rank test, p < 0.0001).
Validity of U22
Cronbach's alpha for the set of U22 scores at baseline, follow-up, and individual patients' score differences were 0.79, 0.94, and 0.91, indicating a satisfactory to excellent internal consistency (). The construct validity of U22 for measurement of symptoms in atrial fibrillation is supported by the relation of the U22 scores to the symptomatic effect of the initial intervention: Improvement in the scores after a clinically inefficient first ablation was significantly smaller than after a singular ablation (). Similarly, a larger improvement in the scores was associated with a significant increase in time to reablation ().
Table III. Estimation of internal consistency of the U22 scores in 105 first-time procedures.
Discussion
Ablation of atrial fibrillation resulted in symptomatic improvement that was recorded by the U22 scores. In the group undergoing repeated procedures, the clinical indication for the reablation is clearly reflected in the diminutive improvement in U22 scores after the first ablation. The latter (≤1 points difference in the U22 scores; ) may be explained by a positive but clinically insufficient effect of the initial ablation or by a placebo effect.
The symptomatic improvement in U22 after the last of repeated ablations is comparable to that after a singular ablation. This is in line with the established clinical experience regarding symptomatic gain from repeated procedures.
The patients' retrospective estimate of improvement at follow-up correlated modestly to the improvement expressed by the difference in the individual scores for well-being between the follow-up form and the baseline form. A retrospective estimation of improvement is therefore at best a rough measure for the change in well-being after ablation of atrial fibrillation.
Cronbach's alpha indicated an excellent internal consistency for the set of U22 scores at follow-up and for the individual patients' score differences between baseline and follow-up. Also the consistency of the U22 scores in the baseline measurement was satisfactory ().
The correlation between U22 scores and SF-36 scores was weak to moderate. U22 is directed towards measurement of arrhythmia symptoms and their effect on well-being. SF-36, on the other hand, is a generic measurement of quality of life and mirrors aspects of atrial fibrillation other than U22.
The present patients with atrial fibrillation have a greater impairment of well-being and higher level of symptoms both before and after an ablation than patients undergoing first-time ablation of AV-nodal re-entry tachycardia or accessory pathway and evaluated by the U22 protocol (Citation18).
Study limitations
We use incidence of reablation at review time as a clinically relevant hard end-point. Due to the nature of atrial fibrillation, relapses occur even after successful ablations. It is therefore not entirely correct to interpret reablation as a sign of an unsuccessful first-time procedure. Added co-morbidity and progress of the arrhythmia might shift a decision towards conservative treatment, in spite of significant symptomatology. Some patients may have developed an asymptomatic permanent atrial fibrillation after the initial ablation. They would not be reablated, and their first-time ablation will thus be considered as successful. Formally this may be correct, since ablation of atrial fibrillation is performed on symptomatic indication. Most cardiologists would nevertheless hesitate to consider such an ablation as successful. These factors may decrease the specificity of reablation as a marker for continued symptoms. They should, however, not affect the conclusion that the symptom quantification by U22 is related to the independent decision regarding a reablation.
In conclusion, U22 measures the symptomatic effect of left atrial ablation for atrial fibrillation. U22 has several practical advantages. It consists of a limited number of questions. The scales have a high resolution (scored 0–10), while many of the other symptom protocols only use four levels. The U22 scores are well suited for a statistical comparison between groups of arrhythmia patients and easily clinically interpreted in individual patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hindricks G, Piorkowski C, Tanner H, Kobza R, Gerds-Li JH, Carbucicchio C, et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation. 2005;112:307–13.
- Bubien RS, Knotts-Dolson SM, Plumb VJ, Kay GN. Effect of radiofrequency catheter ablation on health-related quality of life and activities of daily living in patients with recurrent arrhythmias. Circulation. 1996;94:1585–91.
- Anselme F, Saoudi N, Poty H, Douillet R, Cribier A. Radiofrequency catheter ablation of common atrial flutter: significance of palpitations and quality-of-life evaluation in patients with proven isthmus block. Circulation. 1999;99:534–40.
- Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36:1303–9.
- Jenkins LS, Brodsky M, Schron E, Chung M, Rocco T Jr, Lader E, et al. Quality of life in atrial fibrillation: the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149:112–20.
- Meissner A, Stifoudi I, Weismuller P, Schrage MO, Maagh P, Christ M, et al. Sustained high quality of life in a 5-year long term follow-up after successful ablation for supra-ventricular tachycardia. Results from a large retrospective patient cohort. Int J Med Sci. 2009;6:28–36.
- Walfridsson U, Arestedt K, Stromberg A. Development and validation of a new Arrhythmia-Specific questionnaire in Tachycardia and Arrhythmia (ASTA) with focus on symptom burden. Health Qual Life Outcomes. 2012;10:44.
- Arribas F, Ormaetxe JM, Peinado R, Perulero N, Ramirez P, Badia X. Validation of the AF-QoL, a disease-specific quality of life questionnaire for patients with atrial fibrillation. Europace. 2010;12:364–70.
- Braganca EO, Filho BL, Maria VH, Levy D, de Paola AA. Validating a new quality of life questionnaire for atrial fibrillation patients. Int J Cardiol. 2010;143:391–8.
- Harden M, Nystrom B, Kulich K, Carlsson J, Bengtson A, Edvardsson N. Validity and reliability of a new, short symptom rating scale in patients with persistent atrial fibrillation. Health Qual Life Outcomes. 2009;7:65.
- Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, et al. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:15–25.
- Wokhlu A, Hodge DO, Monahan K, Haroldson J, Wock KJ, Asirvatham SJ, et al. Abstract 633: Unique AF-specific symptom score assesses long-term symptom relief after ablation. Circulation. 2008;118:S589a.
- Dorian P, Paquette M, Newman D, Green M, Connolly SJ, Talajic M, et al. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J. 2002;143:984–90.
- Dorian P, Cvitkovic SS, Kerr CR, Crystal E, Gillis AM, Guerra PG, et al. A novel, simple scale for assessing the symptom severity of atrial fibrillation at the bedside: the CCS-SAF scale. Can J Cardiol. 2006;22:383–6.
- Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC, et al. Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork and the European Heart Rhythm Association. Europace. 2007;9:1006–23.
- Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F Jr, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–41.
- Kesek M, Tollefsen T, Höglund N, Rönn F, Näslund U, Jensen SM. U22, a protocol to quantify symptoms associated with supraventricular tachycardia. Pacing Clin Electrophysiol. 2009;32:S105–8.
- Kesek M, Ronn F, Tollefsen T, Hoglund N, Naslund U, Jensen SM. Symptomatic improvement after catheter ablation of supraventricular tachycardia measured by the arrhythmia-specific questionnaire U22. Ups J Med Sci. 2011;116:52–9.
- Ware JE Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51:903–12.
