Abstract
Thrombocytopenia can cause diagnostic challenges in patients who have received heparin. Heparin-induced thrombocytopenia (HIT) is often considered in the differential diagnosis, and a positive screening can be mistaken as confirmation of the disorder. We present two patients who both received low-molecular-weight heparin for several days. In the first patient, clinical judgment rejected the suspicion of HIT despite a positive screening assay, and treatment for the alternative diagnosis of post-transfusion purpura was correctly initiated. In the second patient, the inaccurate diagnosis HIT was pursued due to a positive screening assay, while the alternative diagnosis of drug-dependent thrombocytopenia caused by piperacillin/tazobactam was rejected. This resulted in re-exposure to piperacillin/tazobactam which caused a second episode of severe thrombocytopenia. A positive screening assay for platelet factor 4/heparin-antibody should be verified by a functional assay, especially in patients with low pretest probability for HIT.
Introduction
Patients developing thrombocytopenia during treatment with heparin or low-molecular-weight heparin for more than four days can pose a diagnostic challenge. If these patients have no other obvious reason for a rapid decrease in platelet counts, such as platelet consumption in severe bleeding or sepsis, an immunological cause is the most likely reason for thrombocytopenia (Citation1). The accurate differential diagnosis between the antibody-mediated procoagulant syndrome of heparin-induced thrombocytopenia (HIT) and other antibody-mediated thrombocytopenic bleeding disorders is important, as management strategies are diametrically opposed (Citation2).
The situation is especially difficult as commercially available antigen assays for detection of antibodies against platelet factor 4 (PF4)/heparin complexes—the cause of HIT—show a poor specificity for clinically relevant PF4/heparin antibodies (Citation3,4). HIT is usually caused by high-titer IgG antibodies directed against a complex of PF4 and heparin. IgA, IgM, and low-titer IgG antibodies directed against the PF4/heparin complexes usually do not cause HIT, but can occur in up to 75% of patients in some patient cohorts (Citation5). This can result in a positive PF4/heparin antigen assay caused by non-pathogenic, non-platelet-activating antibodies. These test results are often misleading and can even distract the clinician from the correct alternative diagnosis. As only IgG antibodies to PF4/heparin activate platelets (Citation3), the risk for an erroneous interpretation of results is especially high with antigen assays recognizing IgG, IgA, and IgM antibodies combined. Clinical judgment, preferably with the use of clinical pretest scores such as the ‘4 T' score (assessing degree of thrombocytopenia, timing of platelet count fall, thrombosis or other sequelae, and other causes of thrombocytopenia (Citation6)) or the recently proposed HIT Expert Probability score (HEP Score) (Citation7), and the HIT Simple Scoring System (Citation8) should be applied before screening tests for antibodies directed against PF4/heparin complexes are performed.
We demonstrate two patients who presented with thrombocytopenia and a positive PF4/heparin antigen test, but a low clinical likelihood for HIT. In the first patient application of systematic scoring systems for clinical HIT almost immediately led to the right non-HIT diagnosis. In contrast, clinical judgment in the second patient resulted in a misdiagnosis of HIT, although use of a systematic score could have guided the clinician towards an alternative diagnosis.
Case presentations
All unnecessary patient details such as sex and exact age were omitted to prevent identification. Approval for publication has been obtained from the ethics committees responsible for Greifswald University Hospital (patient 1) and Uppsala University Hospital (patient 2), respectively.
Case 1
Clinical presentation
An elderly patient presented to the emergency department with fever and pancytopenia (platelets 23 G/L, hemoglobin 4.9 mmol/L, white blood cells 1.8 G/L) caused by oral methotrexate. Methotrexate was administered for rheumatoid arthritis and was immediately withdrawn. Imipenem was started to treat febrile neutropenia, and the patient received two red blood cell concentrates and one platelet concentrate. Subsequently the platelet count rose from 23 to 69 G/L, and on day 2 low-molecular-weight heparin for thrombosis prophylaxis, fluconazole for fungus-induced mucositis, and amiodarone for supraventricular tachycardia were started.
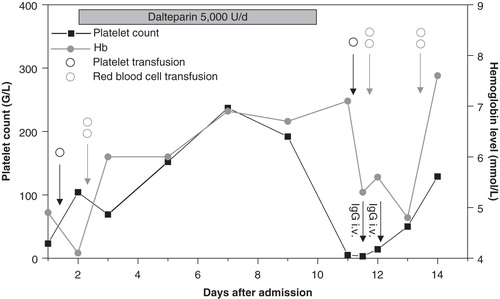
On day 9 the patient developed fever, tachycardia, and hypotension, and the platelet count fell from 234 to 2 G/L (). For possible sepsis-associated thrombocytopenia antibiotic treatment with ciprofloxacin was initiated. On the same day, the patient developed diffuse, transfusion-dependent bleeding from peripheral and central line punctures. Due to the low platelet count one platelet concentrate was given. This caused a febrile transfusion reaction but no platelet count increment after 1 hour.
Differential diagnosis and treatment
The treating physician suspected HIT due to the fall in the platelet count. However, the 4 T score was only 3 (platelet count nadir <10 G/L, onset between days 5 and 10, no new thrombosis, and other causes for thrombocytopenia (such as drug-dependent or sepsis) were possible). The HEP Score was 1 (magnitude of fall >50%, fall began 5–10 days after heparin exposure, nadir ≤20 G/L, presence of bleeding, and newly initiated non-heparin medication known to cause thrombocytopenia), and the Simple Scoring System was 0 (antibody-mediated thrombocytopenia as a significant competing cause for thrombocytopenia). Therefore, the pretest probability for HIT was low, and other causes for thrombocytopenia had to be considered.
Thrombotic thrombocytopenic purpura and disseminated intravascular coagulation can also cause low platelet counts but were promptly excluded due to the absence of fragmented red cells, elevated fibrinogen, and only slightly elevated D-dimer levels, respectively. The abrupt fall in the platelet count and the absence of a platelet count increase 1 hour after transfusion of a platelet concentrate suggested an antibody-mediated disorder. Post-transfusion purpura, drug-dependent thrombocytopenia, and autoimmune thrombocytopenia were considered. Antibiotics are relatively frequent causes of drug-dependent thrombocytopenia. However, as thrombocytopenia occurred rapidly after ciprofloxacin was given, drug-dependent thrombocytopenia to this drug was regarded as very unlikely, and ciprofloxacin was continued. Since a febrile reaction after transfusion of platelet concentrates is typical for post-transfusion purpura, this disorder was considered most likely. However, in the acute situation no clear diagnosis could be made on clinical grounds. As the patient showed severe bleeding, intravenous IgG (1 g/kg body weight) was administered to treat post-transfusion purpura and/or autoimmune thrombocytopenia. Furthermore, all drugs started since admission of the patient to the hospital were withdrawn to interrupt the immune response in potential drug-dependent thrombocytopenia. This resulted in cessation of bleeding within 1 day and recovery of the platelet count within 3 days.
Subsequent laboratory testing
Laboratory testing by an antigen test revealed PF4/heparin IgM antibodies at a low optical density (0.56), while no IgG or IgA antibodies were found. A glycoprotein-specific assay confirmed the presence of strongly reacting alloantibodies against the human platelet antigen-1a, establishing the diagnosis of post-transfusion purpura (Citation9). Furthermore, Pseudomonas aeruginosa sensitive to ciprofloxacin was found in the blood cultures from day 9. Septic shock caused by Pseudomonas aeruginosa may have contributed to the clinical presentation, especially the high temperature, which is typical for neither post-transfusion purpura nor drug-dependent thrombocytopenias.
Case report 2
Clinical presentation
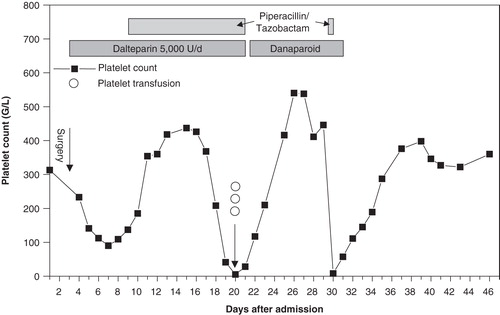
An elderly patient without relevant medical history presented with a traumatic fractured neck of femur (day 1, ). On day 3 a hip endoprosthesis was inserted. During surgery the patient developed hypoxemia and hypotension, which eventually necessitated admission to the critical care unit. Cement or fat embolism was suspected, and the patient received one dose of enoxaparin 40 mg post-surgery (no heparin was given before surgery). Thrombosis prophylaxis was changed to dalteparin 5000 units once daily from day 5 onwards. On day 10 piperacillin/tazobactam was commenced for pneumonia. From day 18 to day 20, the platelet count fell from 208 G/L to 5 G/L. The patient developed petechiae on the trunk, while no clinical signs of thromboembolism were present.
Differential diagnosis and treatment
HIT or piperacillin/tazobactam-induced thrombocytopenia was suspected, and both piperacillin/tazobactam and dalteparin were stopped. Three platelet concentrates were transfused at a platelet count of 5 G/L without an increment. A particle-gel immunoassay (PaGIA, DiaMed GmbH, Cressier, Switzerland) for anti-PF4/heparin antibodies was positive, which seemed to be compatible with the diagnosis of HIT, and danaparoid 2 × 750 units s.c. was started for thrombosis prophylaxis. Subsequently the platelet count rose to a stable plateau until day 30, which seemed to confirm HIT. At that time the patient again developed fever, and piperacillin/tazobactam was restarted at a platelet count of 446 G/L. The next day the platelet count fell to 8 G/L although the patient still received danaparoid, and the alternative diagnosis of piperacillin/tazobactam-induced thrombocytopenia was reconsidered. Both piperacillin/tazobactam and danaparoid were withdrawn and the platelet count recovered over the following 5 days.
HIT was initially suspected by the treating physician due to the fall in the platelet count. As in case 1 the 4 T score was low at only 2 points (platelet count nadir <10 G/L, onset after day 10, no new thrombosis, and other causes for thrombocytopenia (such as drug-dependent) were possible). The HEP Score was 0 (magnitude of fall >50%, fall 11–14 days after heparin exposure, nadir ≤20 G/L, presence of bleeding, and newly initiated non-heparin medication known to cause thrombocytopenia) and the Simple Scoring System was also 0 (antibody-mediated thrombocytopenia as a significant competing cause for thrombocytopenia). Therefore, the pretest probability for HIT was low in three scoring systems, and other causes for thrombocytopenia should have been considered.
Subsequent laboratory testing
Further laboratory testing revealed strongly reactive piperacillin-dependent platelet antibodies, whereas the anti-PF4/heparin-antibody ELISA was positive for anti-PF4/heparin IgA antibodies only, but negative for anti-PF4/heparin IgG or IgM antibodies. The functional assay for platelet-activating clinically relevant HIT antibodies (heparin-induced platelet activation test) (Citation10) was negative.
The assay indicating drug-dependent antibodies (Citation2), the anti-PF4/heparin-antibody ELISA (Citation3), and the heparin-induced platelet activation (HIPA) (Citation10) test were performed as described elsewhere.
Discussion
Patients who develop sudden and/or severe thrombocytopenia without an obvious reason during their hospital stay can cause a diagnostic and management dilemma. These patients have often received unfractionated heparin or low-molecular-weight heparin for several days, but also several other new drugs and/or transfusion of blood products. Whereas in HIT a high risk of thrombosis caused by increased thrombin generation mandates alternative anticoagulation despite low platelet counts (Citation11), other thrombocytopenias are associated with an increased risk of severe bleeding (Citation2). The timing of platelet count decrease is a very important parameter to differentiate a normal early platelet count decrease after major surgery from immune-mediated thrombocytopenias (Citation1). While a platelet count nadir until day 3 after major orthopedic or cardiac surgery is normal and should be expected, a rapid decrease in platelet counts that begins after day 4, and after the platelet count has already started to increase again, is typical for immune-mediated causes in the absence of other causes such as sepsis (Citation1).
In both patients, the drop in platelet counts began 8 and 13 days, respectively, after the start of low-molecular-weight heparin. In patient 1, the platelet count started falling 8–9 days after the first transfusions and after exposure to novel drugs. In patient 2, the platelet count fall started 6 days after the start of antibiotic treatment and almost 2 weeks after surgery. In both patients the platelet count fall was within the typical time frame for immune-mediated thrombocytopenias (drug-dependent thrombocytopenia, HIT, and post-transfusion purpura). HIT is the most frequent of these three causes. It occurs in 0.2% of patients treated with low-molecular-weight heparin after hip replacement surgery, according to a meta-analysis of orthopedic and surgical patients (Citation12). Piperacillin-induced immune thrombocytopenia on the other hand is rare, and only a few cases (Citation13-17) and a recent series of 13 patients have been reported (Citation18). Interestingly, Rousan et al. reported a patient with sepsis and disseminated intravascular coagulation (DIC) in whom HIT or piperacillin-induced immune thrombocytopenia were also considered (and the patient was shown to have the latter). In contrast to our patient (case 2) they were able to rule out HIT by a negative PF4/heparin ELISA (Citation18). Post-transfusion purpura is even rarer than drug-dependent thrombocytopenias, and fewer than 300 cases have been reported (Citation19).
Even if thrombocytopenia is a common feature of HIT, platelet count values <20 G/L do not usually occur in HIT, but are typical for drug-dependent thrombocytopenia and post-transfusion purpura (Citation20). The bleeding signs observed in both patients (diffuse bleeding and petechiae, respectively) also favored the diagnosis of non-HIT-mediated thrombocytopenia.
In clinical practice it is often difficult to weigh all signs and symptoms in a given thrombocytopenic patient. In this regard assessment of the patient by a systematic score for HIT, e.g. the 4 T score (Citation6), can be helpful to guide further assessment and diagnosis. In fact, the 4 T score in both patients was only 2 and 3, respectively, out of 8, ranking them in the low pretest probability group where laboratory testing might not even be necessary (Citation21). In addition, both patients had a low probability for HIT in the HEP and the Simple Scoring System. The particle-gel immunoassay for anti-PF4/heparin-antibodies (patient 2) as well as the anti-heparin/PF4 ELISA (both patients) has a high sensitivity but low specificity for clinical HIT (Citation22). Their strength is to rule out HIT when negative. However, as demonstrated by the present cases, reliance on a positive antigen assay alone can lead to a wrong suspicion or even a misdiagnosis of HIT. In patient 2 the erroneous diagnosis of HIT even caused a second period of severe thrombocytopenia due to re-exposure to the causing antibiotic, luckily without major bleeding complications.
These cases underscore that a positive antigen assay in patients with suspicion of HIT needs further clinical assessment, especially if there is a low pretest probability of HIT, which is particularly important if the test is not IgG-specific (Citation23). Suitable methods for verification are functional assays, which are the only assays that can demonstrate the platelet-activating properties of HIT-antibodies (non-activating low-titer IgG-antibodies seem to lack clinical significance).
Conclusion
In patients with pronounced thrombocytopenia and clinical features favoring other diagnoses than HIT, management can be misguided by a positive HIT-antibody assay. We recommend the use of a systematic approach for the diagnosis of HIT, i.e. using a clinical score such as the 4 T score for HIT as well as further laboratory evaluation of a positive antigen test by a functional assay, especially in patients with low pretest probability of HIT.
Acknowledgements
We are grateful to Dr Daniel Lahner, Medical University of Vienna, Austria, for his thoughtful comments and critical revision of the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Greinacher A, Selleng K. Thrombocytopenia in the intensive care unit patient. Hematology Am Soc Hematol Educ Program. 2010;2010:135–43.
- Greinacher A, Eichler P, Lubenow N, Kiefel V. Drug-induced and drug-dependent immune thrombocytopenias. Rev Clin Exp Hematol. 2001;5:166–200.
- Greinacher A, Juhl D, Strobel U, Wessel A, Lubenow N, Selleng K, et al. Heparin-induced thrombocytopenia: a prospective study on the incidence, platelet-activating capacity and clinical significance of antiplatelet factor 4/heparin antibodies of the IgG, IgM, and IgA classes. J Thromb Haemost. 2007;5:1666–73.
- Lo GK, Sigouin CS, Warkentin TE. What is the potential for overdiagnosis of heparin-induced thrombocytopenia? Am J Hematol. 2007;82:1037–43.
- Selleng S, Malowsky B, Strobel U, Wessel A, Ittermann T, Wollert HG, et al. Early-onset and persisting thrombocytopenia in post-cardiac surgery patients is rarely due to heparin-induced thrombocytopenia, even when antibody tests are positive. J Thromb Haemost. 2010;8:30–6.
- Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759–65.
- Cuker A, Arepally G, Crowther MA, Rice L, Datko F, Hook K, et al. The HIT Expert Probability (HEP) Score: a novel pre-test probability model for heparin-induced thrombocytopenia based on broad expert opinion. J Thromb Haemost. 2010;8:2642–50.
- Messmore HL, Fabbrini N, Bird ML, Choudhury AM, Cerejo M, Prechel M, et al. Simple scoring system for early management of heparin-induced thrombocytopenia. Clin Appl Thromb Hemost. 2011;17:197–201.
- Kiefel V, Santoso S, Weisheit M, Müeller-Eckhardt C. Monoclonal antibody-specific immobilization of platelet antigens (MAIPA): a new tool for the identification of platelet-reactive antibodies. Blood. 1987;70:1722–6.
- Eichler P, Budde U, Haas S, Kroll H, Loreth RM, Meyer O, et al. First workshop for detection of heparin-induced antibodies: validation of the heparin-induced platelet-activation test (HIPA) in comparison with a PF4/heparin ELISA. Thromb Haemost. 1999;81:625–9.
- Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:340S–80S.
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106:2710–15.
- Yan MT, Chu HY, Chau T, Lin SH. Profound thrombocytopenia associated with piperacillin in a hemodialysis patient. Clin Nephrol. 2009;72:240–3.
- Kumar A, Choudhuri G, Aggarwal R. Piperacillin induced bone marrow suppression: a case report. BMC Clin Pharmacol. 2003;3:2.
- Lin SY, Huang JC, Shen MC, Chuang SH, Lee MH, Chen HC. Piperacillin-induced thrombocytopenia reversed by high-flux hemodialysis in an uremic patient. Hemodial Int. 2012;16:S50–3.
- Perez-Vazquez A, Pastor JM, Riancho JA. Immune thrombocytopenia caused by piperacillin/tazobactam. Clin Infect Dis. 1998;27:650–1.
- Olivera E, Lakhani P, Watanakunakorn C. Isolated severe thrombocytopenia and bleeding caused by piperacillin. Scand J Infect Dis. 1992;24:815–17.
- Rousan TA, Aldoss IT, Cowley BD Jr, Curtis BR, Bougie DW, Aster RH, et al. Recurrent acute thrombocytopenia in the hospitalized patient: sepsis, DIC, HIT, or antibiotic-induced thrombocytopenia. Am J Hematol. 2010;85:71–4.
- Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108:759–69.
- Lubenow N, Eichler P, Albrecht D, Carlsson LE, Kothmann J, Rossocha WR, et al. Very low platelet counts in post-transfusion purpura falsely diagnosed as heparin-induced thrombocytopenia. Report of four cases and review of literature. Thromb Res. 2000;100:115–25.
- Hron G, Greinacher A. Advances in the treatment of heparin-induced thrombocytopenia: latest clinical data. Clin Invest. 2011;1:1301–14.
- Bakchoul T, Giptner A, Najaoui A, Bein G, Santoso S, Sachs UJ. Prospective evaluation of PF4/heparin immunoassays for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost. 2009;7:1260–5.
- Lindhoff-Last E, Gerdsen F, Ackermann H, Bauersachs R. Determination of heparin-platelet factor 4-IgG antibodies improves diagnosis of heparin-induced thrombocytopenia. Br J Haematol. 2001;113:886–90.