Abstract
Background. Senile systemic amyloidosis (SSA) derived from wild-type transthyretin is a fairly common condition of old individuals, particularly men. The main presentation is by cardiac involvement, which can lead to severe restrictive cardiomyopathy. SSA is, however, a systemic disease, and amyloid deposits may appear in many other tissues but are thought to be without clinical symptoms outside the heart. Amyloid is a very common finding in cartilage and ligaments of elderly subjects, and transthyretin has been demonstrated in some deposits. Lumbar spinal stenosis is also a condition of usually elderly individuals in whom narrowing of the lumbar spinal canal leads to compression of nerves to the lower limbs.
Results. We questioned whether lumbar spinal stenosis sometimes could be a manifestation of undiagnosed SSA. In this first report we have studied the presence of amyloid in material obtained at surgery for spinal stenosis in 26 patients. Amyloid was found in 25 subjects. Transthyretin was demonstrated immunohistochemically in 5 out of 15 studied resected tissues. Four of the positive materials were analyzed with Western blot revealing both full-length transthyretin (TTR) and C-terminal TTR fragments, typically seen in SSA.
Conclusion. We conclude that lumbar spinal stenosis quite frequently may be a consequence of SSA and that further studies are warranted.
Introduction
Lumbar spinal stenosis is a clinical syndrome of usually elderly individuals that depends on narrowing of the lumbar spinal canal (Citation1). It is characterized by compression of sensory and motoric nerves to the lower limbs, leading to an often disabling condition (Citation1,2). The pathogenesis is probably heterogeneous but includes disc degeneration with disc height decrease and secondary facet-joint subluxation leading to osteoarthritis. Also a degenerative spondylolisthesis of the affected spinal segment may be involved in most cases. Other central factors are general degenerative processes in cartilage and ligaments, including ligamentum flavum. Treatment of lumbar spinal stenosis is often surgical at which thickened connective tissue and osteophytes are removed, leading to a neural decompression and in most cases clinical improvement (Citation3).
The nature of degenerative processes in connective tissue such as ligamentum flavum is poorly understood but obviously associated with aging. A number of studies have shown that amyloid deposits are very common in many ligaments, tendons, and cartilages in elderly individuals (Citation4-6). The importance of such deposits is at present unknown.
Amyloidosis is a generic name for a very diverse group of protein folding disorders, all characterized by creation of cross-beta-sheet fibrils. At least 30 different human proteins have been shown to form amyloid fibrils in vivo (Citation7). Two main groups of amyloid conditions exist: systemic and localized. In the systemic conditions, deposits occur in many organs and tissues, and the diseases are usually life-threatening; in each of these diseases one out of at least 15 plasma proteins forms amyloid fibrils far from the place of parent protein synthesis. In the localized conditions, the proteins are expressed at the site of deposition (Citation8). In both groups, fibrils usually deposit extracellularly and can form conspicuous masses that deform a tissue and interfere with its normal functions.
Joint cartilage and ligaments are targets of both localized and systemic amyloid. Of the systemic forms, Aβ2-microglobulin [for nomenclature, see (Citation7)] amyloidosis is well-known to engage skeletal and joint structures in patients under hemodialysis due to renal insufficiency (Citation9-13). A direct interaction between the fibril protein and collagen I and II seems to be important for distribution of deposits (Citation14). Also immunoglobulin light chain (AL) amyloidosis is known to generate a variety of symptoms from joints and skeleton, sometimes with neural lesions. Carpal tunnel syndrome is often noted in transthyretin (ATTR) and Aβ2-microglobulin amyloidosis (Citation15–17).
Systemic amyloidoses are comparably rare and are included among orphan diseases in Europe. In contrast, site-specific localized forms of amyloid are very common. Thus, medin (AMed) amyloid deposits are seen in the aorta of almost every individual above 60 years. Some, but not all, localized amyloids are associated with specific diseases. Well-known examples are Aβ amyloid deposits in brain in Alzheimer's disease and islet amyloid polypeptide (IAPP) derived deposits seen in the islets of Langerhans in most subjects with type 2 diabetes (Citation8); these two forms have been studied extensively. In contrast, fairly little is known regarding localized amyloid forms in joints and ligaments, and most studies have been focused on prevalence. Particularly knee (Citation5) and hip (Citation4,18–21) joints as well as intervertebral discs (Citation6,19,22) and ligamentum flavum (Citation23,24) have been studied in this respect.
Senile systemic amyloidosis (SSA), derived from wild-type transthyretin (TTR), is common in association with aging, although symptom-giving disease usually is comparably rare and affects males at least 10 times more often than women. Restrictive cardiomyopathy is the main clinical expression. However, carpal tunnel syndrome is common in SSA, and widely spread wild-type ATTR amyloid deposits at other connective tissue sites have been demonstrated (Citation25). Since there are a few case reports on the occurrence of ATTR deposits in the spinal canal we hypothesized that such deposits may be a cause of lumbar spinal stenosis. We therefore collected removed material from patients undergoing surgery for this disease and analyzed it for presence and nature of amyloid.
Material and methods
Resected material was obtained from 26 patients undergoing surgery for lumbar spinal stenosis, and this was the single inclusion criteria. Material consisting of bone fragments and pieces of ligament and other connective tissue was put in 0.15 M NaCl and brought to the laboratory. After rinsing in 0.15 M NaCl followed by distilled water, soft tissue parts, including ligament, were dissected out and washed again. Small pieces were put on a microscopic slide, cut into small pieces with a pair of scissors and thoroughly squeezed against another slide. After separation, the two slides were air-dried and stained with alkaline Congo red (Citation26). Tissue pieces were also fixed in buffered neutral formalin and embedded in paraffin for immunohistochemical studies. Material from two of the patients was re-fixed in glutaraldehyde-paraformaldehyde and embedded in epon (Citation27). Residual material was stored frozen.
Immunohistochemistry and immunoelectron microscopy
Immunohistochemistry was performed in all cases where there was sufficient soft tissue material containing amyloid deposits. Deparaffinized sections were incubated with antiserum 1898 against TTR50-127. This antiserum recognizes both full-length TTR and C-terminal fragments (Citation28), typical of SSA, while many commercially available antibodies do not (Citation29). Other sections were incubated with antiserum 159 or 167 for identification of apolipoprotein A-1 (apoA-I) (Citation30) or with mouse monoclonal antibody Sne5 (Citation31) for detection of protein AA. After application of relevant secondary antibodies, immunoreaction was visualized with 3,3’-diaminobenzidine-tetrahydrochloride. Immunoelectron microscopy with antiserum 1898 was performed as described (Citation27), and immunoreaction was shown with 10 nm gold particles.
Proximity ligation assay
Since both TTR and apoA-I have been described as amyloid fibril proteins in joints and ligaments, proximity ligation assay (PLA) was performed with monoclonal antibodies to TTR (mouse monoclonal prealbumin clone E-1, Sc 377517, Santa Cruz, CA, USA) and rabbit antiserum to apoA-I (A159). PLA was executed according to the manufacturer's (Olink Bioscience, Uppsala, Sweden) instructions. As positive control two different antibodies against TTR (monoclonal antibodies as above and polyclonal 1898) were applied.
Western blot analysis
Fragments from ligaments were cut into small pieces, washed thoroughly in 0.15 M NaCl followed by distilled water and finally acetone, and air dried. Materials were extracted with sample buffer, and Western blot with the primary rabbit antisera 1898 (against TTR) and 159 and 167 (against apoA-I) was performed as described (Citation32). Reaction was visualized with an enhanced chemiluminescence system (GE Healthcare, Uppsala, Sweden).
Ethical considerations
This study is of pilot character, and since we did not know whether any results would be generated, we decided to perform the investigations on de-identified materials. Therefore we only have obtained sex and age of patients. This study is in accordance with Swedish law (the Ethical Committee at Uppsala University Hospital was consulted).
Results
Typical amyloid deposits were identified microscopically in 21 of the 26 specimens (). The morphological appearance varied considerably. Some deposits were diffuse, weakly congophilic, and not demarcated, while others were distinct and often showed strong affinity for Congo red. The latter commonly appeared as scattered small lesions. In some cases, many distinct and often larger deposits were found, particularly in tissue of ligament structure.
Table I. Total material analyzed for presence of amyloid from patients with lumbar spinal stenosis.
Material was suitable for immunohistochemistry in 15 cases (). There was a weak but inconsistent reaction for TTR in some of the diffuse deposits. Many amyloid areas were completely negative with this antiserum. However, in five cases, distinct and uniform TTR immunoreactivity was seen in many deposits. These were strongly congophilic and exhibited a typical green birefringence. Double staining with TTR immunohistochemistry followed by Congo red (Citation26) showed complete overlapping (). Electron microscopy of material from two cases with amyloid deposits showing TTR positivity at immunohistochemistry revealed amyloid fibrils of typical appearance at close proximity to collagen and elastin (). Such fibrils were immunolabeled with anti TTR50-127 antiserum ().
Figure 1. Section of ligament with amyloid deposits immunolabeled for transthyretin and stained with Congo red. Overlapping of immunoreaction with Congo red positivity is evident. Polarized light with partially crossed polars. Bar 200 µm.
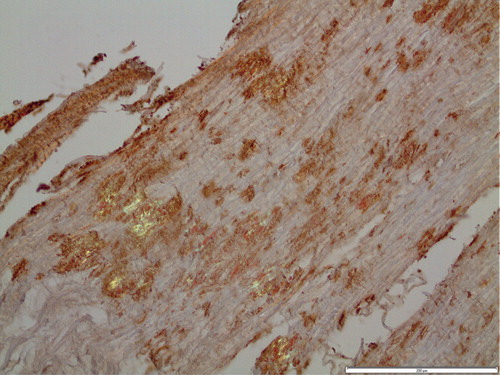
Figure 2. Electron micrograph of ligament with transthyretin-amyloid deposits (A) with typical fine fibrillar appearance, intermingled with collagen fibers (Co). The section was immunolabeled for transthyretin and reaction visualized with 10 nm gold particles. Bar 500 nm.
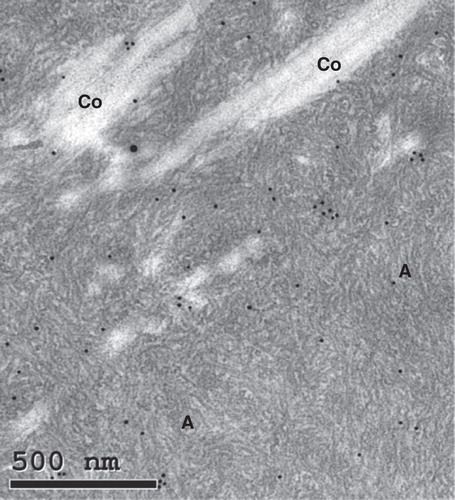
Table II. Amyloid-containing materials with or without reactivity with an anti TTR antibody at immunohistochemistry.
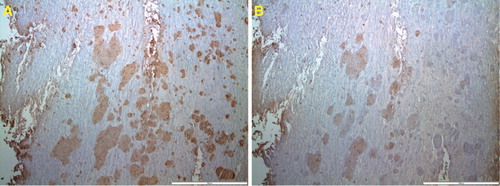
Since apoA-I has been found in amyloid of knee joints (Citation33), immunohistochemistry was performed with an apoA-I antiserum in 10 of the cases with amyloid deposits, with or without TTR immunoreactivity. In two of the studied materials a partial apoA-I immunolabeling of TTR-positive amyloid was found (). This finding raised the question whether amyloid fibrils with mixed composition occurred, and therefore PLA with one monoclonal antibody against TTR and one polyclonal antiserum against apoA-I were applied on sections of one of the apoA-I immunopositive cases. The result was completely negative, indicating that apoA-1 is not an integrated part of the ATTR amyloid fibril. The positive control with monoclonal and polyclonal antibodies against TTR gave a strong signal as expected.
Figure 3. Adjacent sections of ligament immunolabeled for transthyretin (A) and apoA-I (B). While immunolabeling for transthyretin is homogeneous, that for apoA-I is more spotty. Bar 200 µm.
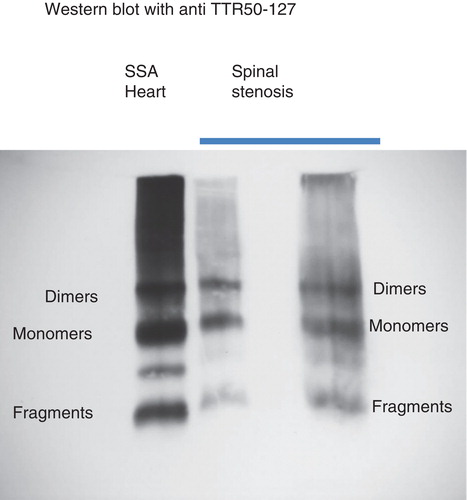
Western blot analysis was performed with amyloid-rich TTR-positive material from four subjects (3 males and 1 female). Strong bands corresponding to TTR full-length monomers and C-terminal fragments, typical of ATTR in SSA (Citation27), were obtained in all cases (exemplified in ). These patients were 82, 76, 72, and 77 years old, respectively (mean 76.7 years). As a control, material was tested from one 59-year-old woman with immunohistochemically TTR-negative amyloid deposits. No bands appeared at Western blot analysis.
Figure 4. Western blot analysis of extracts of two spinal stenosis materials, positive for transthyretin at immunohistochemistry. Included is also an extract of amyloid fibrils obtained from the heart of a patient with senile systemic amyloidosis. The pattern is the same in all three materials with full-length transthyretin as well as a prominent band corresponding to C-terminal fragments. The unlabeled band between monomers and dimers has constantly been found in senile systemic amyloidosis and may be dimers of fragments.
Discussion
Very high prevalence figures have been reported for local amyloid deposits in several different joints and ligaments. However, it has been difficult to evaluate the clinical importance of these often very small deposits, and their genesis is completely unknown. The amyloid has generally been believed to be of localized nature and of limited interest, partially depending on the high prevalence. There have been attempts to analyze the nature of the amyloid, usually by immunohistochemistry with the aid of antibodies against known amyloid fibril proteins. Very few direct biochemical studies have been performed. In one investigation, based on formaldehyde-fixed material from knee joint meniscus, apoA-1 was demonstrated by mass spectrometry of extracts of amyloid deposits (Citation33). There was, however, no TTR found. Results of a number of immunohistochemical studies have been partially contradictory. In several immunohistochemical studies TTR immunoreactivity has been demonstrated in amyloid deposits in various ligaments and articular tissues. In one paper 17 out of 50 hip joints showed amyloid deposits, and all were reported to be labeled with antibodies against TTR (Citation20). Sueyoshi et al. studied 36 specimens from patients with lumbar canal stenosis and found amyloid in material from 19 individuals. In 16 of these amyloid was of a TTR nature (Citation34). Those with amyloid deposits were older than those without. The same group reported one patient in whom development of SSA was highly suspected (Citation35). No further studies of the amyloid protein were performed.
Our results show that small amyloid deposits are extremely common in the tissues around the spinal canal, not only in aged subjects. Quite evidently, these amyloid deposits may be of heterogeneous biochemical nature, and TTR is the major component in relatively few. Interestingly, individuals with ATTR amyloid were comparably old in the present investigation, similarly to findings reported in a previous study (Citation34), which strengthens the suspicion of a relationship with SSA. TTR in plasma is a homotetrameric protein consisting of four identical about 14-kDa subunits. In amyloid of all patients with SSA (and in a majority of those with hereditary ATTR amyloidosis), fibrils are mainly composed of C-terminal TTR fragments, in SDS-PAGE appearing as a broad, about 8-kDa, band below the usually minor full-length TTR band (Citation27,36). Such a fragment band was demonstrated in three of the four materials analyzed by Western blot. Although it cannot be ruled out that localized ATTR deposits with this composition may occur, this electrophoretic pattern can be an indication that deposits around the spinal canal are part of SSA.
SSA is known to manifest itself as a severe form of cardiomyopathy of elderly men (Citation37), but it is a systemic disease with deposits in many organs (Citation25,38). Renal involvement is not rare although underdiagnosed and may be of clinical relevance (Citation38). Carpal tunnel syndrome is commonly associated with connective tissue deposition of amyloid and has been suggested sometimes to be a first sign of SSA (Citation39). Carpal tunnel syndrome has been reported to occur up to decades before other symptoms of disease in mutant ATTR amyloidosis, such as the Danish TTR L111M mutation (Citation40). From our present results it seems very likely that spinal stenosis is another not uncommon result of SSA. Therefore, cardiac evaluation with echocardiography may be warranted in patients with spinal stenosis, at least in elderly men.
Another interesting and potentially important question is whether there is an increased risk of amyloid-generated spinal stenosis in hereditary amyloidosis of TTR origin. Hereditary ATTR amyloidosis is phenotypically highly heterogeneous and often clinically overlapping with SSA, e.g. in cardiac and carpal tunnel manifestations. Further studies of this possibility are necessary.
Acknowledgements
Thanks are due to Ellahe Charkhar and Marianne Ljungquist for skilled assistance.
Declaration of interest: Supported by the Swedish Research Council and the FAMY, FAMY Norrbotten, and Amyl foundation. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Katz JN, Harris MB. Lumbar spinal stenosis. N Engl J Med. 2008;358:818–25.
- Suri P, Rainville J, Kalichman L, Katz JN. Does this older adult with lower extremity pain have the clinical syndrome of lumbar spinal stenosis? JAMA. 2010;304:2628–36.
- Sasaji T, Horaguchi K, Yamada N, Iwai K. Improvement of hip abductor muscle weakness after lumbar decompressive surgery. Ups J Med Sci. 2012;117:426–9.
- Ladefoged C, Christensen HE. Congophilic substance with green dichroism in hip joints in autopsy material. Acta Pathol Microbiol Scand A. 1980;88:55–8.
- Ladefoged C. Amyloid deposits in the knee joint at autopsy. Ann Rheum Dis. 1986;45:668–72.
- Ladefoged C, Fedders O, Petersen OF. Amyloid in intervertebral discs: a histopathological investigation of surgical material from 100 consecutive operations on herniated discs. Ann Rheum Dis. 1986;45:239–43.
- Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, et al. Amyloid fibril protein nomenclature: 2012 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid. 2012;19:167–70.
- Westermark P. Amyloid in the islets of Langerhans: thoughts and some historical aspects. Ups J Med Sci. 2011;116:81–9.
- Bardin T, Kuntz D, Zingraff J, Voisin M-C, Zelmar A, Lansaman J. Synovial amyloidosis in patients undergoing long-term hemodialysis. Arthritis Rheum. 1985;28:1052–8.
- Gejyo F, Yamada T, Odani S, Nakagawa Y, Arakawa M, Kunitomo T, et al. A new form of amyloid protein associated with chronic hemodialysis was identified as b2-microglobulin. Biochem Biophys Res Commun. 1985;129:701–6.
- Gorevic PD, Casey TT, Stone WJ, DiRaimondo CR, Prelli F, Frangione B. Beta-2 microglobulin is an amyloidogenic protein in man. J Clin Invest. 1985;76:2425–9.
- Munoz-Gómez J, Bergadá-Barado E, Gómez-Pérez R, Llopart-Buisán E, Subías-Sobrevía E, Rotés-Querol J, et al. Amyloid arthropathy in patients undergoing periodical haemodialysis for chronic renal failure: a new complication. Ann Rheum Dis. 1985;44:729–33.
- Heegaard NH. β2-Microglobulin: from physiology to amyloidosis. Amyloid. 2009;16:151–73.
- Giorgetti S, Rossi A, Mangione P, Raimondi S, Marini S, Stoppini M, et al. β2-Microglobulin isoforms display an heterogeneous affinity for type I collagen. Protein Sci. 2005;14:696–702.
- Kyle RA, Eilers SG, Linscheid RL, Gaffey TA. Amyloid localized to tenosynovium at carpal tunnel release. Natural history of 124 cases. Am J Clin Pathol. 1989;91:393–7.
- Yamamoto S, Kazama JJ, Narita I, Naiki H, Gejyo F. Recent progress in understanding dialysis-related amyloidosis. Bone. 2009;45:S39–42.
- M’Bappé P, Grateau G. Osteo-articular manifestations of amyloidosis. Best Pract Res Clin Rheumatol. 2012;26:459–75.
- Ladefoged C. Amyloid in osteoarthritic hip joints: deposits in relation to chondromatosis, pyrophosphate, and inflammatory cell infiltrate in the synovial membrane and fibrous capsule. Ann Rheum Dis. 1983;42:659–64.
- Foitschik T, Saeger W, Riebe M, Röcken C. Advanced glycation end products in intervertebral discs and hip joint capsules: correlation with senile amyloid? Amyloid. 2005;12:167–73.
- Niggemeyer O, Steinhagen J, Deuretzbacher G, Zustin J, Rüther W. Amyloid deposition in osteoarthritis of the hip. Arch Orthop Trauma Surg. 2011;131:637–43.
- Niggemeyer O, Steinhagen J, Fuerst M, Zustin J, Rüther W. Amyloid deposition in rheumatoid arthritis of the hip. Rheumatol Int. 2012;32:2645–51.
- Mihara S, Kawai S, Gondo T, Ishihara T. Intervertebral disc amyloidosis: histochemical, immunohistochemical and ultrastructural observations. Histopathology. 1994;25:415–20.
- Gies U, Linke RP, Schachenmayr W. Amyloid deposits of immunohistochemically different classes in the ligamentum flavum in biopsies from patients with herniated discs or lumbar spinal stenosis. Clin Neuropathol. 1996;15:54–9.
- D’Agostino AN, Mason MS, Quinn SF. Lumbar spinal stenosis and spondylosis associated with amyloid deposition in the ligamentum flavum. Clin Neuropathol. 1992;11:147–50.
- Pitkänen P, Westermark P, Cornwell GG III. Senile systemic amyloidosis. Am J Pathol. 1984;117:391–9.
- Westermark GT, Johnson KH, Westermark P. Staining methods for identification of amyloid in tissue. Methods Enzymol. 1999;309:3–25.
- Bergström J, Gustavsson Å, Hellman U, Sletten K, Murphy CL, Weiss DT, et al. Amyloid deposits in transthyretin-derived amyloidosis: cleaved transthyretin is associated with distinct amyloid morphology. J Pathol. 2005;206:224–32.
- Bergström J, Murphy CL, Weiss DT, Solomon A, Sletten K, Hellman U, et al. Two different types of amyloid deposits - apolipoprotein A-IV and transthyretin - in a patient with systemic amyloidosis. Lab Invest. 2004;84:981–8.
- Ihse E, Ybo A, Suhr OB, Lindqvist P, Backman C, Westermark P. Amyloid fibril composition is related to the phenotype of hereditary transthyretin V30M amyloidosis. J Pathol. 2008;216:253–61.
- Mucchiano GI, Häggqvist B, Sletten K, Westermark P. Apolipoprotein A-1-derived amyloid in atherosclerotic plaques of the human aorta. J Pathol. 2001;193:270–5.
- Nyström SN, Westermark GT. AA-Amyloid is cleared by endogenous immunological mechanisms. Amyloid. 2012;19:138–45.
- Ihse E, Suhr OB, Hellman U, Westermark P. Variation in amount of wild-type transthyretin in different fibril and tissue types in ATTR amyloidosis. J Mol Med. 2011;89:171–80.
- Solomon A, Murphy CL, Kestler DP, Coriu D, Weiss DT, Makovitzky J, et al. Amyloid contained in the knee joint meniscus is formed from apolipoprotein A-I. Arthritis Rheum. 2006;54:3545–50.
- Sueyoshi T, Ueda M, Jono H, Irie H, Sei A, Ide J, et al. Wild-type transthyretin-derived amyloidosis in various ligaments and tendons. Hum Pathol. 2011;42:1259–64.
- Sueyoshi T, Ueda M, Sei A, Misumi Y, Oshima T, Yamashita T, et al. Spinal multifocal amyloidosis derived from wild-type transthyretin. Amyloid. 2011;18:165–8.
- Ihse E, Rapezzi C, Merlini G, Ando Y, Suhr OB, Ikeda S, et al. Amyloid fibrils containing fragmented ATTR may be the standard fibril composition in ATTR amyloidosis. Amyloid. 2013;20:142–50.
- Johansson B, Westermark P. Senile systemic amyloidosis: a clinico-pathological study of twelve patients with massive amyloid infiltration. Int J Cardiol. 1991;32:83–92.
- Westermark P, Bergström J, Solomon A, Murphy C, Sletten K. Transthyretin-derived senile systemic amyloidosis: clinicopathologic and structural consideration. Amyloid. 2003;10 Suppl 1: 48–54.
- Sekijima Y, Uchiyama S, Tojo K, Sano K, Shimizu Y, Imaeda T, et al. High prevalence of wild-type transthyretin deposition in patients with idiopathic carpal tunnel syndrome: a common cause of carpal tunnel syndrome in the elderly. Hum Pathol. 2011;42:1785–91.
- Svendsen IH, Steensgaard-Hansen F, Nordvåg BY. A clinical, echocardiographic and genetic characterization of a Danish kindred with familial amyloid transthyretin methionine 111 linked cardiomyopathy. Eur Heart J. 1998;19:782–9.

