Abstract
Antimicrobial susceptibility testing with phenotypic methods requires breakpoints, i.e. a minimum inhibitory concentration (MIC) categorizing micro-organisms into susceptible, intermediately susceptible, and resistant for the relevant antimicrobial agent. Determinations of breakpoints require tools such as the understanding of dosing, MIC distributions of organisms without resistance mechanisms, pharmacokinetics, pharmacodynamics, and of clinical outcome in defined clinical situations. Several European countries (France, Germany, Norway, Sweden, the Netherlands, and UK), have national breakpoint committees, often with 20–30 years of experience and tradition. These committees now co-operate under the umbrella of the European Committee on Antimicrobial Susceptibility Testing (EUCAST), organized by The European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the European Centre for Disease Prevention and Control (ECDC). Together with the European Medicines Agency (EMA), EUCAST determines breakpoints for existing and new antibacterial and antifungal agents. Moreover, EUCAST has developed a disk diffusion antimicrobial susceptibility testing method which is now, together with the new European breakpoints, being implemented in many countries both inside and outside Europe.
Introduction
Antimicrobial susceptibility testing (AST) of bacteria and fungi is performed to predict the outcome of antimicrobial chemotherapy of infections in patients, to determine the local epidemiology of antimicrobial resistance to form a basis for empirical therapy, to detect and thus provide opportunities to prevent the spread of organisms carrying especially unwanted resistance mechanisms (infection control and public health), and to measure the rate of the development of resistance and correlate this to activities which may increase or decrease resistance development.
Measuring the susceptibility of micro-organisms
Susceptibility of micro-organisms can be measured with phenotypic and/or genotypic methods. The phenotypic methods, such as determining the minimum inhibitory concentration (MIC) value of an antimicrobial agent for the organism, can predict both sensitivity and resistance, whereas genotypic methods predict resistance only. Using phenotypic methods, resistance and sensitivity are quantifiable. An organism inhibited or killed by a very low concentration of the agent is categorized as more sensitive than one which is not inhibited or killed even by a high concentration. The latter will most certainly be categorized as clinically resistant, whereas to decide how organisms without resistance mechanisms or with only low-level resistance should be categorized is a much more complex decision. This needs to build on a formal gathering of experts (a breakpoint committee) with competences in microbiology, infectious diseases, pharmacology, and pharmacodynamics. Following a period with a multitude of individual initiatives a few colleagues came forth to lead. Among the founding fathers of susceptibility testing were Ericsson and Sherris (Citation1) and Bauer and coworker(Citation2). The many initiatives created a problem; systems were developed in France, Germany, the Netherlands, Sweden, the UK, the USA, and Norway, and all developed in slightly different directions:
BSAC (British Society for Antimicrobial Chemotherapy, UK; http://www.bsac.org.uk).
CA-SFM (Comité de l'ántibiogramme de la Société Française de Microbiologie, France; http://www.sfm.asso.fr).
CLSI, originally the NCCLS (Clinical Laboratory Standards Institute, USA; http://www.clsi.org).
CRG (Commissie Richtlijnen Gevoeligheids-bepalingen, the Netherlands).
DIN (Deutsches Institut für Normung, Germany).
NWGA (Norwegian Working Group on Antibiotics, Norway; http://www.unn.no/category10274.html).
SRGA and SRGA-M (Swedish Reference Group of Antibiotics, Sweden, and its subcommittee on methodology; http://www.srga.org).
The concentration that separates sensitive from non-sensitive micro-organisms is called the S-breakpoint and is expressed as S≤X mg/L (where X is a MIC value), and the concentration which separates resistant organisms from non-resistant (e.g. sensitive or intermediately sensitive) organisms is called the R-breakpoint and is expressed as R>Y mg/L (where Y may be the same or a higher MIC value than X). Only by random chance did the committees agree on a breakpoint. shows cefotaxime and gentamicin breakpoints for Enterobacteriaceae prior to any effort to harmonize. Both illustrate the surprising differences between committees in their definitions of breakpoints. The right-hand column shows the effect on the measurement of resistance to gentamicin in Escherichia coli of having different breakpoints. Depending on which breakpoint is used, resistance will be reported as a rate between 1.9% and 14.3%. Note also that what is deemed resistant (R) by one committee could be categorized as sensitive (S) by another committee.
Table I. Differences in breakpoints between seven breakpoint committees before the harmonization process.
The national committees in Europe had, with few exceptions, only national followers. Each country spent much time on developing and upholding their systems. The 2–4 yearly meetings of each committee were closed meetings and involved the 10–15 committee members. Almost all committees developed not only breakpoints but also a more or less unique disk diffusion method.
This method—i.e. the placement of antibiotic-containing paper disks (although other materials were tried) on an inoculated agar plate and the reading of the diameter of inhibition zones as a surrogate measurement of the MIC—developed in several directions. The European committees based their disk diffusion methods on the International Collaborative Study (Citation1), and all used a semi-confluent inoculum but different media. In the US, the NCCLS (later CLSI) was formed and based their recommendations on the publications of Bauer et al. (Citation2), where a confluent inoculum was recommended. The recommendations from the CLSI became widely used also outside the US. CLSI meets twice a year and consists of 12 voting members and 12 advisors. Since 2002 the author has been the European advisor. The voting members are from the medical profession, from government agencies, such as the centre for disease control (CDC), and from the private sector, both pharmaceutical industry and industry manufacturing AST material. The meetings are traditionally open to all, and the meeting room is often crowded with spectators, mostly from industry interested in following the proceedings.
So in effect the world had, until 2002, at least seven different interpretative systems for testing bacteria for susceptibility to antibiotics. Comparison of antimicrobial resistance development was often difficult unless one compared rates of methicillin resistant staphylococcus aureus (MRSA), vancomycin resistant enterococci (VRE), beta-lactamase-producing Haemophilus influenza, ESBL-producing Enterobacteriaceae, and a few others, where breakpoints and phenotypic methodology were not critical. This was either because surrogate tests were used or because molecular microbiology was used to detect the resistance gene. Despite these differences and shortcomings, there were only feeble attempts to unite the committees, and no one had the authority to take real action.
The birth of the European Committee on Antimicrobial Susceptibility Testing
In 1997, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) formed the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Initially the committee was set up without the involvement of the six national European committees. It soon became evident that, with this set-up, harmonization of breakpoints could not be achieved—instead of one European committee, there were now seven committees active in Europe.
In 2001, the author was asked to spend time on determining whether or not it would be possible to form a truly European breakpoint committee with the remit to harmonize breakpoints in Europe. By then EUCAST was an expensive operation for ESCMID, and, unless there was a valid plan to achieve European agreement on AST, ESCMID wanted to close down the committee. With some trepidation about work-load and travelling, and only because the scientific secretary Derek Brown (Cambridge, UK) accepted to take this on, there was an agreement. During 12 months the six European committees were visited, and the need for and merits of a joint European breakpoint system were discussed. The organization proposed would not necessarily interfere with the development of the national disk diffusion systems (there were tests from BSAC, CA-SFM, CRG, DIN, and SRGA). Eventually, all six committees pledged their commitment to the common cause. A model for how six national European committees could retain their national structure and importance and still work together was created. Each committee remained, and all of them had one representative in a joint committee. However, they would forthwith not decide on breakpoints except together with the other committees. A steering committee, with a chairman and a scientific secretary (and later a clinical data co-ordinator), one representative from each of the national committees, and two representatives from other European countries, was created. A General Committee, with representatives from all European (and later other) countries, was also founded, and by-laws were agreed upon. The national committees signed contracts binding them to the work ahead. However, the ESCMID Executive Committee hesitated, since it was felt that many countries in Europe were following CLSI and that maybe it was not necessary to create a European system. The national breakpoint committees made it clear that adopting CLSI recommendations was not acceptable and pointed out that Europe lacked all influence on the process of determining CLSI breakpoints and that industry influence was pronounced and unacceptable to Europeans. Also, European systems were all freely available to users, and it would not be possible to advise laboratories to have to buy the recommendations. With some hesitation ESCMID accepted the plans and decided to provide funds for EUCAST committee work. EUCAST was advised to change the acronym for the committee. This advice was not followed, and EUCAST would live on but hopefully with a new reputation. Eventually, funds from EU (EU Commission Public Health Programme 2003) and the European Centre for Disease Prevention and Control (ECDC) (Framework Partnership Agreement between ECDC and ESCMID/EUCAST in 2008 and later in response to several calls for tender up to and including 2014) were applied for and obtained, and since then ECDC, ESCMID and the national breakpoint committees have shared the burden of EUCAST. The revamped EUCAST Steering Committee went to work (Citation3).
Tools for determining breakpoints
Determinations of breakpoints require tools. Fifty years ago breakpoints were based on MICs and serum concentrations. All species had the same breakpoint for an antimicrobial agent. Today indications for use are specific, and only defined species are given breakpoints. Gone are the days when an agent was given breakpoints for ‘infections caused by bacteria sensitive to the agent’. Today breakpoints are mirrored against important resistance mechanisms, and what used to be pharmacokinetics in 10 healthy volunteers is now volunteers and patients and simulated variance in populations of 10,000 or more. The tools have developed (), and what used to be an art is now an art based on science.
Table II. Tools and information defined by EUCAST as necessary for determining breakpoints.
Epidemiological cut-off values (ECOFF), PK/PD cut-offs, and clinical cut-offs will together define a clinical breakpoint. The role of pharmacokinetics and pharmacodynamics in EUCAST breakpoint setting was recently published (Citation4).
MIC distributions of phenotypically wild-type bacteria and fungi
The EUCAST Steering Committee was shown the MIC distribution diagrams of phenotypically wild-type bacteria as used by the SRGA in the late 1980s. It was decided that breakpoint discussions in the committee could not rely on singular MIC distributions brought to the table by an individual or a national committee. There was no agreement on methods 15 years ago, and each committee supported their own. The factual difference between methods was not well known.
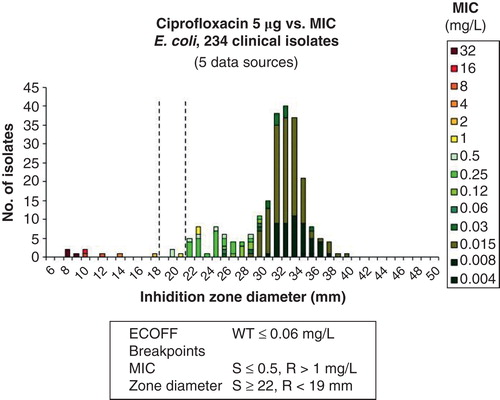
A web-based prototype for collecting large numbers of MIC distributions from many international sources was developed. Very quickly large quantities of MIC values were obtained or spontaneously contributed from investigators all over the world. Today there are more than 25,000 contributions, all in the form of MIC distributions, in the database, and some agent/species distributions consist of 50 individual contributions and more than 50,000 MIC values. The MICs are from human and veterinarian medicine, from large surveillance studies, from scientific projects, food safety programmes, and clinical studies. There are also antifungal distributions in Candida and Aspergillus species. There are now more than 100,000 yearly visits to this freely available website (http://www.eucast.org/mic_distributions/). On the first page of the website, often missed by users, is a description of how the contributions were obtained. On these wild-type distributions of species-specific MIC values from many sources, EUCAST has determined the agent/species-specific epidemiological cut-off value (ECOFF). These are now used in many different programmes, in human medicine, veterinary medicine, and food safety. Most ECOFFs were determined using ‘the eye-ball method’, but also statistical methods were developed to calculate the ECOFF (Citation5). ,, show some typical graphs (as exemplified for ciprofloxacin) from the MIC and inhibition zone distribution website, and shows a graph from the EUCAST ‘calibration and validation’ website.
Figure 1. EUCAST ciprofloxacin MIC distributions from the EUCAST website (http://mic.eucast.org/Eucast2/SearchController/search.jsp?action=performSearch&BeginIndex=0&Micdif=mic&NumberIndex=50&Antib=47&Specium=-1).
Figure 2. E. coli ciprofloxacin MIC distribution from the EUCAST website, as a result of clicking on ‘E. coli’ in Figure 1 (http://mic.eucast.org/Eucast2/regShow.jsp?Id=1022).
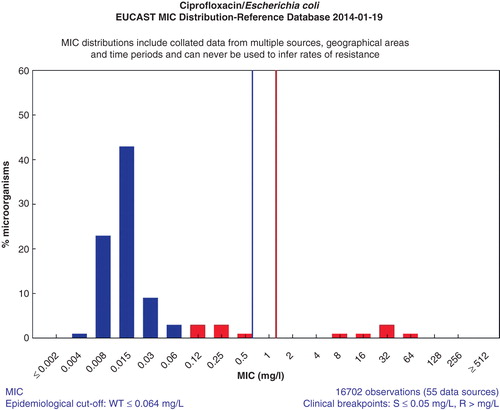
Figure 3. E. coli inhibition zone diameter distribution as a result of changing view from ‘MIC’ to ‘Disk diffusion’ (http://mic.eucast.org/Eucast2/regShow.jsp?Id=26694).
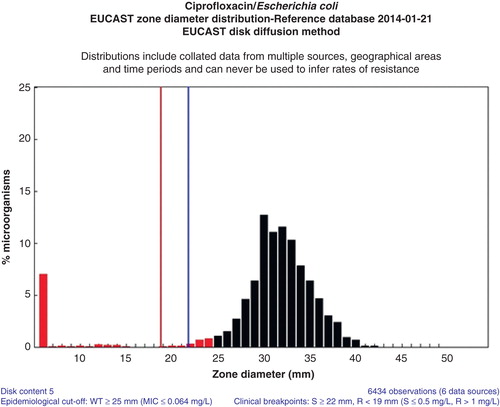
Figure 4. Relationship between E. coli ciprofloxacin MICs and inhibition zone diameters used by EUCAST to determine the correlation between MICs and zone diameters and to determine zone diameter breakpoints. These are available at: http://www.eucast.org/antimicrobial_susceptibility_testing/calibration_and_validation/.
Completion of the harmonization process
Between 2002 and 2010, European breakpoints for all relevant existing agents were harmonized by EUCAST. For each agent, a rationale document describing the harmonization process, listing the data used and any relevant exceptions, was produced and published on the EUCAST website (http://www.eucast.org/documents/rd/).
The harmonized breakpoints were gradually introduced into national systems, and by 2010 there was a complete set of European breakpoints, accepted by European authorities and by colleagues all over Europe. Companies producing susceptibility testing materials and machines gradually developed EUCAST criteria for automated susceptibility testing. This process, which was slow and cumbersome and frustrated many colleagues, disclosed the inherent flaws of the automated systems. All of them lack the flexibility needed for modern susceptibility testing. In a world with very few new antibiotics but hordes of new resistance mechanisms, and where the rapid change of breakpoints to meet new challenges is of utmost importance, it is unacceptable to have to wait sometimes years for new breakpoints.
The EUCAST website
A website (www.eucast.org) was set up under the auspices of ESCMID. At present, it has approximately 20,000 hits per month from all over the world. The website lists all EUCAST recommendations and publications, both for bacteria and fungi, many in several languages, and all rationale documents. It gives access to breakpoint tables for screen and printing. It also provides educational material and links to MIC and inhibition zone diameter distributions. It is free of charge and open to all.
EUCAST subcommittees
Several subcommittees with defined remits and time schedules have been formed over the last 10 years:
The subcommittee on antifungal susceptibility testing sets breakpoints for antifungal agents for Candida spp. and Aspergillus spp. It also provides standard methodology for both organisms (http://www.eucast.org/antifungal_susceptibility_testing_afst/).
The subcommittee on anaerobe susceptibility testing (currently not active) prepared discussions on breakpoints for Gram-positive and Gram-negative anaerobes.
The subcommittee on expert rules and interpretive reading in susceptibility testing (currently not active) has published a much-cited article in which the evidence for various expert rules, often used by microbiologists, is scrutinized and categorized. A typical rule is: ‘If a Staphylococcus aureus is shown to be methicillin-resistant then report the organism as resistant to all other beta-lactam agents’ (Citation6). The rules change over time and need regular updating.
The subcommittee on the detection of resistance mechanisms of clinical and/or public health interest (currently not active) was instituted to help colleagues to decide when susceptibility testing can be performed with breakpoints only (and be reported as tested) and when it is important to detect, identify, and report a resistance mechanism, irrespective of whether or not the organism is categorized as resistant. This document is available on the website (http://www.eucast.org/resistance_mechanisms/).
Subcommittees that are currently not active have been dissolved because they completed the task given to them. The subcommittee on antifungal susceptibility testing, with a steering committee of appointed experts and a general committee with country representatives, continues its work.
Agreement with EMA
An agreement was forged with the European Medicines Agency (EMA; London, UK) and the pharmaceutical industry. A standard operation procedure (SOP) regulating EUCAST's role in the setting of breakpoints for antimicrobial agents undergoing registration was agreed upon. It is available on the websites of both EMA and EUCAST. The pharmaceutical industry is welcome to present or discuss their agents with EUCAST before, during, and after the registration process. As part of the regulatory process for registering new drugs, EUCAST, together with EMA, has set breakpoints for daptomycin, tigecycline, and ceftaroline, to mention a few.
EUCAST, EU, and ECDC
EUCAST obtained grants from the European Union (EU) to support the process of harmonizing European breakpoints. As all other EU-funded ‘networks’, EUCAST was to be incorporated in the European Centre for Disease Prevention and Control (ECDC), Stockholm, Sweden, once the ECDC had been formed. However, the structure and remit of EUCAST did not fit the frame of the other EU-funded networks (such as the previously mentioned European Resistance Surveillance System (EARSS) which became EARS-Net). EUCAST was given an ‘outside role’ and has responded to ECDC calls for tender for the determination of European breakpoints. EUCAST also provides technical and expert advice to ECDC in general and EARS-Net in particular. This is on antimicrobial susceptibility testing, external quality assessment (EQA), and the need and methods for detection, categorization, and reporting of resistance mechanisms of clinical and/or public health importance.
The EUCAST disk diffusion test
Following the results of a questionnaire distributed in 2009 to clinical microbiology departments throughout Europe, EUCAST decided to develop a disk diffusion test built on a platform already known to most colleagues in Europe, the Mueller Hinton medium with a confluent McFarland 0.5 inoculum. This is similar to the CLSI recommendations. The lack of enthusiasm for the CLSI-recommended medium for Haemophilus influenzae and the fact that the CLSI system required two different plates to deal with Basingstoke, streptococci and H. influenzae, prompted us to develop a medium which could be used for both streptococci and H. influenzae, and, as it turned out, several other fastidious organisms. We had experience from a medium used in Sweden and the UK for H. influenzae and streptococci including Staphylococcus pneumoniae, Isosensitest medium (Oxoid Ltd, Thermo Fisher Scientific, Basingstoke, UK) supplemented with 5% horse blood and beta-NAD. Isosensitest was exchanged for Mueller Hinton medium (MH), and this medium (MH-F) could be validated for use with H. influenzae, streptococci, and many other fastidious micro-organisms. The three CLSI media were reduced to two media in the EUCAST method.
It was reasoned that European countries already using CLSI methodology, especially medium and inoculum preparation, would more easily adopt European recommendations if the leap was less dramatic. The aim was to make sure that European countries were all using the same breakpoints. Without a common susceptibility test this would be difficult to achieve.
ESCMID decided to take the financial responsibility for developing the disk diffusion test. Work commenced in 2010. A network of laboratories from all over the world was created, with the Växjö laboratory in a co-ordinating role. Great support for the initiative was gained from many quarters. It was decided to base all recommendations on data obtained with MH and disks from several manufacturers. Broth micro-dilution (BMD) plates, specific to our needs and manufactured to the specifications of the ISO document (Citation7), were produced. Collections of modern isolates were offered with characterized resistance mechanisms. Producing parallel MIC and zone diameter data, the EUCAST laboratory created thousands of MIC/zone diameter correlations such as the one shown in and available on the EUCAST website (http://www.eucast.org/antimicrobial_susceptibility_testing/calibration_and_validation/). These are used to properly calibrate the disk diffusion zone diameter breakpoints to the clinical MIC breakpoints. The robustness of the method is constantly tested by provoking it and the zone diameter breakpoints by adding many isolates with ‘borderline’ resistance. The EUCAST disk diffusion test was recently described (Citation8) and evaluated in accordance with ISO recommendations (Citation9,10).
Since 2010 the EUCAST disk diffusion method has been adopted by one country after the other. Today all Nordic countries have adopted EUCAST methods and breakpoints, and so have Estonia, Croatia, Poland, Italy, Austria, Switzerland, Slovenia, the Netherlands, France, and many other European countries, but also Australia and countries in Africa. Questions to EUCAST are posed from all parts of the world including the US, South-east Asia, Canada, and South America, clearly indicating the international interest in the EUCAST initiative.
Participation of countries outside Europe
The structure and by-laws of EUCAST have been changed to allow input from outside Europe. Countries were encouraged to form national antimicrobial susceptibility testing committees (NACs) and to join the EUCAST General Committee. More than 25 countries have already heeded the call, including countries far outside Europe, such as Australia, South Africa, and the US. Many of the NACs, including the NACs from Australia and the US, have presented themselves on the EUCAST website (http://www.eucast.org/organization/nac/).
In CLSI, the structure and business model remain largely unchanged. ‘The Blue Books’ are famous both for their structured content and for the money it costs to acquire them. The influence of industry, both pharmaceutical industry and manufacturers of antimicrobial susceptibility testing devices and material, remains the same.
EUCAST in 2014 and onwards
For a few years EUCAST has been on variable time contracts with ECDC, Stockholm, Sweden. EUCAST responds to calls for tender. ECDC finances the committee work of EUCAST and its subcommittees. EUCAST provides advice to ECDC, EMA, and European Food Safety Agency (EFSA) on matters related to susceptibility testing, the setting of breakpoints and ECOFFs, detection of resistance, the measuring of resistance rates, and external quality assessment of antimicrobial susceptibility testing. For ECDC we monitor the implementation of EUCAST breakpoints and methods in European countries. During 2013 several separate polls have indicated that, over the last 2–3 years, the transition from previous systems to EUCAST breakpoints have gone from 30% to 70% of laboratories. ESCMID has agreed to take long-term financial responsibility for the EUCAST disk diffusion test.
International harmonization of breakpoints and methods
Following a joint decision and with a memorandum of understanding, EUCAST and CLSI have formed an ad hoc subcommittee on ‘Determination of methods and breakpoints for polymyxin/colistin’ with the intention of arriving at harmonized international breakpoints for the polymyxins. This is of importance since to an increasing number of patients this drug is the last resort in infections caused by multidrug-resistant isolates. The symbolic value of co-operation between the world's breakpoint committees is important.
The EUCAST disk diffusion method is built on the same platform as the disk diffusion method recommended by CLSI, which means that for many agent/species combinations the inhibition zone diameter obtained in the test can be interpreted against either the EUCAST or the CLSI breakpoint table.
Whether or not these are successful steps towards a future further international harmonization remains to be seen. As we move into 2014 the French committee recommends French laboratories to abandon the CA-SFM disk diffusion method and to adopt the EUCAST method. Only the UK still upholds its Isosensitest-based disk diffusion method (calibrated to EUCAST clinical breakpoints), but laboratories in Wales and Scotland have adopted the EUCAST method and an increasing number of laboratories in the UK too.
Conclusions
Antimicrobial susceptibility testing in Europe is evidently harmonized, although it will take some time before all laboratories have managed the transition. Whether or not global harmonization is possible is another matter. It could happen because an increasing number of countries and colleagues are attracted to the easy and free availability of EUCAST recommendations and because everyone can be part of the decision process through the open consultations published several times a year on the website (www.eucast.org).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ericsson HM, Sherris JC. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1–90.
- Bauer AW, Perry DM, Kirby WM. Single-disk antibiotic-sensitivity testing of staphylococci; an analysis of technique and results. AMA Arch Intern Med. 1959;104:208–16.
- Kahlmeter G, Brown DF, Goldstein FW, MacGowan AP, Mouton JW, Osterlund A, et al. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J Antimicrob Chemother. 2003;52:145–8.
- Mouton JW, Brown DFJ, Apfalter P, Canton R, Giske CG, Ivanova M, et al. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin Microbiol Infect. 2012;18:E37–45.
- Turnidge J, Kahlmeter G, Kronvall G. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cutoff values. Clin Microbiol Infect. 2006;12:418–25.
- Leclercq R, Canton R, Brown DFJ, Giske C, Heisig P, MacGowan AP, et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin Microbiol Infect. 2013;19:141–60.
- ISO 20776-1 (2006). Clinical laboratory testing and in vitro diagnostic test systems—Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices—Part 1: reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. Available at www.iso.org/iso/iso_catalogue_detail.htm?csnumber=41630.
- Matuschek E, Brown DF, Kahlmeter G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect. 2013; Epub ahead of print.
- ISO 20776-2 (2007). Clinical laboratory testing and in vitro diagnostic test systems—Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices—Part 2: evaluation of performance of antimicrobial susceptibility test devices. Available at www.iso.org/iso/iso_catalogue_detail.htm?csnumber=41631.
- Bengtsson S, Bjelkenbrant C, Kahlmeter G. Validation of EUCAST zone diameter breakpoints against reference broth microdilution. Clin Microbiol Infect. 2013; Epub ahead of print.

