Abstract
Background. Atelectasis is common during and after general anaesthesia. We hypothesized that a ventilation strategy, without recruitment manoeuvres, using a combination of continuous positive airway pressure (CPAP) or positive end-expiratory pressure (PEEP) and a reduced end-expiratory oxygen fraction (FETO2) before ending mask ventilation with CPAP after extubation would reduce the area of postoperative atelectasis.
Methods. Thirty patients were randomized into three groups. During induction and emergence, inspiratory oxygen fractions (FIO2) were 1.0 in the control group and 1.0 or 0.8 in the intervention groups. No CPAP/PEEP was used in the control group, whereas CPAP/PEEP of 6 cmH2O was used in the intervention groups. After extubation, FIO2 was set to 0.30 in the intervention groups and CPAP was applied, aiming at FETO2 < 0.30. Atelectasis was studied by computed tomography 25 min postoperatively.
Results. The median area of atelectasis was 5.2 cm2 (range 1.6–12.2 cm2) and 8.5 cm2 (3–23.1 cm2) in the groups given FIO2 1.0 with or without CPAP/PEEP, respectively. After correction for body mass index the difference between medians (2.9 cm2) was statistically significant (confidence interval 0.2–7.6 cm2, p = 0.04). In the group given FIO2 0.8, in which seven patients were ex- or current smokers, the median area of atelectasis was 8.2 cm2 (1.8–14.7 cm2).
Conclusion. Compared with conventional ventilation, after correction for obesity, this ventilation strategy reduced the area of postoperative atelectasis in one of the intervention groups but not in the other group, which included a higher proportion of smokers.
Introduction
During general anaesthesia, the combination of reduced functional residual capacity, airway closure, and a high inspiratory oxygen fraction (FIO2) are the main factors implicated in the development of atelectasis, shunt and shunt-like effects that account for the majority of the impaired oxygenation seen during general anaesthesia (Citation1,2).
Previous studies have shown that formation of atelectasis during preoxygenation and induction of anaesthesia can be avoided by adding a continuous positive airway pressure (CPAP) followed by a positive end-expiratory pressure (PEEP) (Citation3,4). During emergence from anaesthesia, high concentrations of oxygen (‘postoxygenation’) predispose to atelectasis formation (Citation5). Even a recruitment manoeuvre, followed by ventilation with FIO2 1.0 with a PEEP/CPAP of 10 cmH2O until extubation, failed to improve postoperative oxygenation compared with that achieved with zero end-expiratory pressure (ZEEP) (Citation6). This failure may have been caused by the presence of lung regions with high oxygen concentrations, which are prone to collapse shortly after extubation and the discontinuation of CPAP. We hypothesized that by inducing and discontinuing anaesthesia during CPAP/PEEP and deliberately reducing FIO2 after extubation, postoperative atelectasis would be reduced compared with standard protocols. We also designed the study with two intervention groups differing in oxygen concentration to investigate if, in the longer term, FIO2 of 0.8 confers any benefit over FIO2 1.0 in reducing the area of atelectasis. To test our hypothesis, we studied 1) a control group with no CPAP/PEEP and an FIO2 of 1.0 while breathing spontaneously after extubation; and 2) two intervention groups that were on CPAP/PEEP of 6 cmH2O from induction to extubation, one group receiving an FIO2 of 1.0 and the other FIO2 0.8 until extubation, and then in both groups an FIO2 of 0.3 via a face-mask while on CPAP after extubation. Computed tomography (CT), undertaken in the immediate postoperative period, was used to investigate the degree of atelectasis, and peripheral arterial oxygen saturation (SpO2) at FIO2 0.21 was measured to estimate differences in oxygenation during mechanical ventilation and spontaneous breathing.
Materials and methods
Patients
The Regional Ethics Committee (Uppsala, Sweden, protocol D-nr 2008/251) approved the study (2008-10-23), and all patients (n = 30) gave their written informed consent for participation. The enrolled patients were studied during and after general anaesthesia for eye surgery (Örebro, n = 22) or day-case orthopaedic surgery (Köping, n = 8). Local anaesthesia was given to all patients, eliminating the need for postoperative opioids.
Adult patients aged up to 75 years with American Society of Anesthesiologists (ASA) physical status class I–III were recruited to the study, provided they could climb two flights of stairs without stopping, had an SpO2 of ≥ 94% when breathing air, and a body mass index (BMI; weight in kilograms divided by the square of the height in metres) of <31. Patients with chronic obstructive pulmonary disease, overt heart failure, or for whom a difficult intubation was predicted were not included.
Apparatus and monitoring
The patients' electrocardiogram and SpO2 were continuously monitored during anaesthesia, as were end-tidal CO2 (ETCO2) concentration and end-expiratory oxygen fraction (FETO2), and non-invasive blood pressure was measured intermittently with the Infinity Delta XL (Draeger Medical Ag & Co. KG, Lubeck, Germany) or the Philips IntelliVue MP Anesthesia monitor (Philips Medizin Systeme, Boeblingen, Germany). The Primus Draeger (built-in PEEP: 0 cmH2O) (Draeger Medical Ag & Co. KG) and the Datex-Ohmeda S/5 Avance (built-in PEEP: 3 cmH2O) (GE Healthcare, Datex-Ohmeda, Madison, WI, USA) were used for mechanical ventilation and spontaneous breathing, with both ventilators functioning as circle systems. Neuromuscular function was monitored using a neuromuscular train-of-four (TOF) monitor from Draeger (Medical Ag & Co. KG) or the TOF-Watch S (Organon Ltd, Dublin, Ireland). Target-controlled infusions (TCI) of propofol and remifentanil were delivered by target-controlled infusers, the Care Fusion (Alaris Medical UK Ltd, Basingstoke, UK) or the Agilia Injectomat TIVA (Fresenius Vial, Brezius, France). SpO2 was continuously measured postoperatively with the Infinity Masimo SET (Masimo Corporation, Irvine, CA, USA) or the Ohmeda TuffSat (GE Healthcare Finland Oy, Kuortaneenkatu, Helsinki, Finland).
Anaesthesia
The experimental procedure started with preoxygenation. After 1 min a TCI of propofol was started at 2 μg/mL. Three minutes after the start of preoxygenation, the propofol infusion was increased to 6 μg/mL, and a TCI of remifentanil was started at 4 ng/mL. To facilitate endotracheal intubation, rocuronium was given at 0.5 mg/kg ideal body weight (IBW) (Citation7). During anaesthesia induction, the patients were hydrated with Ringer acetate to reduce the risk of post-induction hypotension. After intubation, the TCI infusions of propofol and remifentanil were reduced to match the level of surgical stimulation, according to the clinical signs of the depth of anaesthesia. Before ending anaesthesia, all patients were checked for residual neuromuscular block and antagonized if the TOF recovery was ≤ 90%.
Randomization
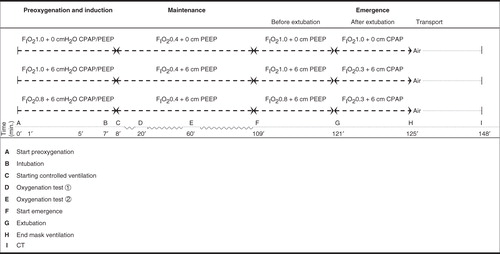
Patients were randomized using the sealed envelope technique to one of three groups: a control group (FIO2 1.0, 0 cmH2O of CPAP/PEEP) and two intervention groups (FIO2 1.0, 6 cmH2O of CPAP/PEEP, and FIO2 0.8, 6 cmH2O of CPAP/PEEP). The overall study design is presented in .
Ventilation during preoxygenation, anaesthesia induction, and maintenance
In the control group, FIO2 was 1.0 during preoxygenation and induction, and no CPAP or PEEP was used at any time. When spontaneous breathing ceased during induction in the control group, the anaesthesiologist inflated the lungs manually. After intubation, the patients were given volume-controlled ventilation (VCV) with ZEEP.
In the intervention groups, FIO2 was either 1.0 or 0.8 during preoxygenation and induction, and the ventilator was used in the pressure support mode with a CPAP of 6 cmH2O. When spontaneous breathing subsided during induction, the ventilator mode was shifted to pressure control ventilation (PCV), and the former CPAP level was transformed automatically to the same PEEP level.
After endotracheal intubation, the patients in the intervention groups were continuously ventilated in PCV with a PEEP level of 6 cmH2O. After confirmation of successful intubation, FIO2 was reduced to 0.40 (in nitrogen) in all groups. The minute ventilation was monitored to keep ETCO2 concentration as constant as possible at approximately 5%, with the respiratory frequency set between 8 and 11 breaths per minute and the tidal volume set to approximately 6 mL/kg IBW. Fresh gas flow was set to 8 L/min during induction and to 1 L/min during maintenance of anaesthesia.
Ventilation during emergence from anaesthesia
After completion of surgery, FIO2 was set to the same level as during anaesthesia induction with a fresh gas flow of 8 L/min in all three groups. The patients in the control group were extubated after establishing spontaneous breathing, and they were given FIO2 1.0 via face-mask after extubation. The patients in the intervention groups were extubated in the same manner as the control group, but they were given a CPAP of 6 cmH2O applied via face-mask immediately after extubation. Within a few seconds, as soon as the anaesthesiologist could confirm a free upper airway, the FIO2 was lowered to 0.30, and spontaneous breathing with CPAP continued, aiming at a FETO2 ≤ 0.30. The duration of mask ventilation after extubation was recorded for each patient.
Ventilation during transport to and in the radiology department
Postoperatively, SpO2 was measured continuously. During this period, all patients were awake and breathed room air spontaneously. Patients were given extra oxygen if they did not fulfil the recommendations of the British Thoracic Society (Citation8), i.e. if the SpO2 was <94%.
Oxygenation
During air breathing, SpO2 was recorded when patients were positioned supine on the operating table just before the start of preoxygenation. Oxygenation was measured on two occasions during maintenance of anaesthesia: firstly, during stable anaesthesia approximately 20 min after the start of preoxygenation and, secondly, under stable anaesthesia conditions approximately 40 min later. The tests were performed after reducing the FIO2 to 0.21 (air). The fresh gas flow was set to 12–18 L/min during the tests, to shorten the time for establishing a new steady state for FETO2. When FIO2 reached 0.21 and a new matching level of FETO2 was established after about 1 min, the observations of FETO2 and SpO2 continued for approximately 5 min.
CT
Aeration and area of atelectasis in the lungs were studied by CT (Philips MX8000 Quad; Philips Medical Systems, Best, the Netherlands, or GE LightSpeed VCT XTe; GE Healthcare, Waukesha, WI, USA) (Citation9). During awakening, the time-frame from the final removal of the face-mask, with the patient still on the operating table, to completion of the CT scan in the Radiology department was measured in all patients. All patients were placed horizontally on the CT table in the supine position with their arms parallel to their body. A basal single-slice transverse scan, 5 mm thick, 10–20 mm above the dome of the right diaphragm was performed for each patient. The scan was located in the proper position following a scouting scan of the lungs. Both the scouting and the basal scan were carried out while the patients held their breath after a normal expiration. A radiologist, blinded to the group affiliation of the patients, measured the area of atelectasis. Calculations of the atelectasis area in each basal scan were made using the region of interest (ROI) program present in the CT computer software. For tracing the area of atelectasis, the window level and width were set at –500 and ±1,500 Hounsfield units, respectively. Atelectasis was defined as an area with attenuation values between –100 and +100 Hounsfield units and was expressed in square centimetres and in percentage of the total area of the lung at the basal scan. The dorsal border was traced manually between the atelectasis and the pleura. The ventral border was drawn a few centimetres above the dense area, and the area of atelectasis was calculated using the software program. Each ROI was redrawn twice and the mean value was used.
Statistical analysis
The main dependent variable, the area of postoperative atelectasis, determined the sample size. Based on earlier studies, it was predicted that a difference of 50% in the area of atelectasis could be achieved between groups, which would require 10 patients in each group, assuming α = 0.05 and 1 – β = 0.8.
Because of the small number of patients, the Mann–Whitney U test was used to test the median difference between groups. The difference in SpO2 between the awake state and during anaesthesia for all patients and the differences between groups during anaesthesia and postoperatively were analysed with the Wilcoxon matched pairs signed rank test.
Results
With the exception of their smoking habits, the groups were similar in demographic and physiological data (). Sixteen patients were given midazolam for premedication (). Time data for specific events during anaesthesia () and ventilatory data during controlled ventilation () were also similar between groups. Two patients in the control group were unintentionally exposed to a PEEP of 3 cmH2O because of the built-in PEEP in the anaesthesia ventilator. All patients spontaneously recovered from the neuromuscular block (TOF = 100%) before extubation.
Table I. Demographic and physiologic data (median and range) for study groups.
Table II. Time data for special events during anaesthesia (median and range) in study groups.
Table III. Ventilatory data with controlled ventilation during maintenance of anaesthesia (median and range) in study groups.
After extubation, there was a period lasting 2–4 min with mask ventilation in all three groups. All patients in the control group breathed FIO2 1.0, and FETO2 was always ≥0.9 at the beginning of mask ventilation. In the intervention groups, all patients breathed FIO2 0.3 during mask ventilation, and FETO2 progressively decreased from an initial level of ≥0.9 (with FIO2 1.0) or ≥0.7 (with FIO2 0.8) at the beginning of mask ventilation.
Atelectasis was investigated approximately 25 min postoperatively. The median areas of atelectasis with and without CPAP/PEEP were 5.2 cm2 (2.4%) (range 1.6–12.2 cm2, 0.7%– 5.1%) and 8.5 cm2 (3.9%) (3–23.1 cm2, 1.7%–10.5%), respectively, in the groups given FIO2 1.0. This median difference of 3.3 cm2 was not statistically significant. The 95% confidence interval (CI) was –0.8 to 8.4 cm2 (p = 0.07). However, after correcting for BMI, using Willett's residual method (Citation10), the median difference was 2.9 cm2 and the 95% CI was 0.2– 7.6 cm2, and the result proved statistically significant (p = 0.04). In a post hoc analysis, after adjusting for age (Willett's residual method), the median difference was 2.8 cm2, and the 95% CI was 0.1–8.4 cm2 (p = 0.03). Finally, after adjusting for both BMI and age (Willett's residual method), performing a post hoc analysis in the area of atelectasis formation between the two groups given an FIO2 of 1.0, the median difference was 3.6 cm2, and the 95% CI was 1.3–9.2 cm2 (p = 0.02) ().
Figure 2. Area of postoperative atelectasis 1 cm above the dome of the right diaphragm in the control and intervention group given FIO2 1.0, unadjusted (raw data) and adjusted for age and BMI. Boxes represent the first to the third quartile. The dark line is the median. Extended bars represent the range. *p = 0.016 (Mann–Whitney U test).
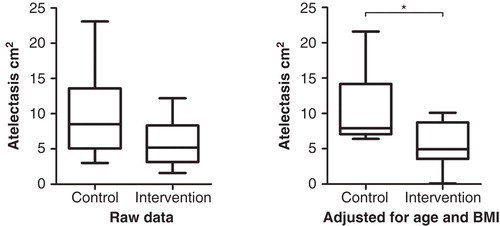
The area of atelectasis in the group given FIO2 0.8 with CPAP/PEEP was 8.2 cm2 (4.4%) (1.8–14.7 cm2, 1.1%–5.8%). Compared with any of the groups given FIO2 1.0, the differences in the area of atelectasis were not statistically significant, even after adjustment for age or BMI or both. In a post hoc analysis with Fisher's exact test, the distribution of ex- or current smokers in the group given FIO2 0.8 differed from the corresponding distribution in the control group given FIO2 1.0 without CPAP/PEEP (p = 0.02). The distribution of ex- or current smokers in the group given FIO2 0.8 did not differ from the corresponding distribution in the other intervention group (p = 0.07). For all three groups the Spearman correlation coefficient for atelectasis and age was 0.46 (p < 0.05) and for atelectasis and BMI 0.16 (p = 0.39).
The median differences in SpO2, observed by comparing values immediately before anaesthesia with values measured twice during ventilation with air during anaesthesia, were statistically significant in all study groups (). There was no difference in SpO2 with air between the three study groups before, during, or after anaesthesia (). Postoperatively, only one patient in either group temporarily needed extra oxygen to achieve a SpO2 ≥ 94%. No patient needed extra oxygen during the CT investigation.
Table IV. Peripheral oxygen saturation (SpO2) (median and range) in study groups measured with FIO2 0.21.
Discussion
This study indicated that a ventilation strategy that aimed to keep the peripheral airways open by using CPAP/PEEP, without the use of a recruitment manoeuvre (RM), reduced the area of postoperative atelectasis by 40%, relatively, in spite of the use of FIO2 1.0 during induction of and emergence from anaesthesia. After a correction for BMI or age, or both, this difference in postoperative atelectasis area was statistically significant.
For all groups, oxygenation was significantly lower when breathing air during anaesthesia than during the awake condition before anaesthesia, but oxygenation did not differ between groups, either during or after anaesthesia.
In the group given 6 cmH2O CPAP/PEEP and FIO2 0.8, the intervention failed to reduce atelectasis. However, the number of previous and current smokers in this group was significantly higher than in the control group given FIO2 1.0 without CPAP/PEEP.
Statistical considerations
This study was planned to detect a difference in the area of atelectasis of at least 50%, so an actual difference of 40% reached a p value of 0.07. The only modifying factor that has previously been proven to increase the area of atelectasis is a high BMI. It was assumed that the randomization procedure would render the impact of BMI equal between groups, but it was not. However, the primary result was so close to being statistically significant, which can be inferred from the confidence interval, that a small and justified correction for the difference in the uneven BMI effect between the two groups given an FIO2 of 1.0 moved the confidence interval and the p value was lowered to 0.04.
That age was also found to be a modifying factor, increasing the area of atelectasis, has not been shown before. Overall, the sample size was too small to accommodate for uneven distribution of known (BMI) or unproven (age, smoking?) effect modifiers, exposing the results to the risk of making a type 2 error.
General considerations
The aim of this study was to evaluate the impact of a comprehensive ventilation strategy, from preoxygenation and induction of anaesthesia to spontaneous breathing after awakening from anaesthesia, on the immediate postoperative area of atelectasis. The ambition was to keep the lung open for the whole perioperative period without relying on an RM. There were three major differences between the control and the intervention groups: 1) the mode of controlled ventilation; 2) the use of CPAP/PEEP; and 3) the level of FIO2. The design of the study therefore precludes any definitive conclusions regarding the separate importance of these factors. However, they were selected based on findings from previous studies and compared with a control group, representing the historical appearances of a conventional ventilation strategy with an FIO2 of 1.0 during preoxygenation, without CPAP/PEEP, with VCV and a high FIO2 during postoxygenation.
Despite a clear effect on atelectasis formation, the preventive effect of our investigated strategy might have been even more obvious had the technique of applying CPAP via a face-mask been more effective. In practice, leaks occurred that could have lowered the airway pressure from the target 6 cmH2O. The leaks also made FETO2 readings at the end of mask ventilation less reliable. It is also possible that our chosen level of CPAP/PEEP of 6 cmH2O was not high enough to keep the airways open in some patients. A CPAP level of 6 cmH2O during induction has been shown to prevent early formation of atelectasis in patients up to a BMI of 27 (Citation4), but we included patients with a BMI up to 31. In a study of anaesthetized patients with a mean BMI of 26.7, only a minor degree of atelectasis was found 10 min after a vital capacity manoeuvre and a PEEP level of 10 cmH2O with 100% oxygen (Citation11). In another study, a PEEP level of 10 cmH2O was found to be optimal with regard to the best compliance and the smallest dead space fraction (Citation12). However, although a PEEP level of 10 cmH2O might be optimal during stable anaesthesia after expanding the lungs, we chose 6 cmH2O to facilitate spontaneous breathing and patient comfort, especially during awakening, and to reduce the risk of hypotension during induction and intubation. We realize that the technique can be improved, and that it might be better to adjust the level of CPAP/PEEP for each patient to optimize prevention of atelectasis.
The impact of BMI and age
We found no significant correlation between BMI and atelectasis, although previous studies found a weak correlation (Citation13,14). It is probable that the use of a CPAP/PEEP of 6 cmH2O reduced the impact of overweight on atelectasis formation. However, because of reduced variance the difference in atelectasis formation between groups became statistically significant after adjusting for BMI.
Age was found to be a risk factor for the formation of atelectasis. To understand this finding, it is necessary to comprehend the relationship between airway closure and age. With increasing age, the amount of airway closure within the tidal volume increases, which is presumably the main explanation for the reduced oxygenation of arterial blood with increasing age (Citation15). One would thus predict more atelectasis in older persons, but this has not been shown previously. The explanation may be that with a greater degree of airway closure, preoxygenation takes a little longer in the older person (Citation16,17). If preoxygenation is short and the minute ventilation low, as could be the case with heavy premedication, older patients will have less denitrogenation of the lungs, which may counteract the collapse of the alveoli behind the closed airways. More efficient denitrogenation using CPAP may thus promote the formation of atelectasis, and if more time is allowed for the older patient to reach the same FETO2 during preoxygenation as the younger patient, atelectasis may become more apparent in the older patient.
Smoking
Smoking was more common in the group given 6 cmH2O CPAP/PEEP and FIO2 0.8, in spite of randomization. A link between smoking in clinically lung-healthy subjects and anaesthesia-induced atelectasis has not been previously described. Therefore, several months after completion of the study, every patient was contacted and a more in-depth interview regarding smoking habits and postoperative respiratory problems was carried out. No patient reported a postoperative respiratory problem. In another recent study we have demonstrated, in a post hoc analysis, that patients smoking more than six pack-years constituted a subgroup of patients with more atelectasis (Citation18).
Oxygenation
The lack of difference between groups in SpO2 postoperatively, despite the difference in the amount of atelectasis, might be because the accuracy of the SpO2 measurement is not sufficiently sensitive to discriminate a small difference in oxygenation. Even though all groups presented some atelectasis, in two patients as much as 10% of the lung CT area in dependent parts of the lungs, almost all patients fulfilled the British Thoracic Society recommendation for SpO2 without being given extra oxygen, and all patients were in good clinical condition. A previous study (Citation5), investigating the effect on postoperative atelectasis of different FIO2 before tracheal extubation, found an even higher level of atelectasis than in our study. All patients in that study also had a SpO2 ≥ 94% while breathing air. As atelectasis consists of compressed lung tissue, the amount of lung that has collapsed during and after ‘uneventful’ anaesthesia is in fact larger than the detected area of atelectasis, close to 15% on average and exceeding 30%–40% in some cases (Citation1). It is also notable that a SpO2 of 94% when breathing air can be observed with a shunt fraction of approximately 15%, assuming ‘normal’ values for the other variables in the shunt equation (Citation19,20). This will go unnoticed if SpO2 is the only variable monitored.
Limitations of the study
The recommendation for optimal preoxygenation differs, but a period of 3 min is often advocated (Citation21). We chose to preoxygenate for only 1 min, as this is in fact a common procedure when not anticipating a difficult induction. A longer period of preoxygenation should increase the alveolar oxygen concentration, thus making the lungs more vulnerable to early collapse (Citation22). Reducing the time for preoxygenation probably influenced alveolar oxygen concentration more in the control group than in the intervention groups, since no CPAP was used in the control group. This, if anything, should reduce the difference in atelectasis between the control and study groups. More atelectasis might have been observed in the controls if preoxygenation had been longer.
The control group was given VCV during maintenance of anaesthesia, as opposed to PCV in the intervention groups. The ventilation mode in the control group was chosen to make this group comparable with previous studies. The ventilation mode in the intervention group was chosen for practical purposes, as it made the transition from spontaneous breathing to controlled ventilation easy, setting the right values for PCV from the preceding values for pressure support ventilation and CPAP. No advantage has been shown per se for PCV over VCV (Citation23,24), and we believe that the difference in ventilation mode had little influence on the results.
In summary, we have shown that a ventilation strategy with CPAP/PEEP and FIO2 1.0 during induction of and emergence from anaesthesia, followed by CPAP and low oxygen concentration via a face-mask after extubation, was successful in reducing the amount of postoperative atelectasis in non-smoking patients. CPAP is relatively simple to implement during induction of anaesthesia but might be more difficult after extubation.
Acknowledgements
We would like to thank Anders Nydahl, MD, PhD, Chief of Staff and Lena Lundgren, CRNA, Department of Anaesthesiology and Intensive Care, University Hospital, Örebro, Sweden for their support and skilful assistance.
Declaration of interest: This study was financially supported by intramural departmental funding (both Departments of Anaesthesiology), and from the Centre for Clinical Research. The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hedenstierna G, Edmark L. Mechanisms of atelectasis in the perioperative period. Best Pract Res Clin Anaesthesiol. 2010;24:157–69.
- Hedenstierna G, Rothen HU. Respiratory function during anesthesia: effects on gas exchange. Compr Physiol. 2012;2:69–96.
- Coussa M, Proietti S, Schnyder P, Frascarolo P, Suter M, Spahn DR, et al. Prevention of atelectasis formation during the induction of general anesthesia in morbidly obese patients. Anesth Analg. 2004;98:1491–5.
- Rusca M, Proietti S, Schnyder P, Frascarolo P, Hedenstierna G, Spahn DR, et al. Prevention of atelectasis formation during induction of general anesthesia. Anesth Analg. 2003;97:1835–9.
- Benoit Z, Wicky S, Fischer JF, Frascarolo P, Chapuis C, Spahn DR, et al. The effect of increased FIO2 before tracheal extubation on postoperative atelectasis. Anesth Analg. 2002;95:1777–81.
- Lumb AB, Greenhill SJ, Simpson MP, Stewart J. Lung recruitment and positive airway pressure before extubation does not improve oxygenation in the post-anaesthesia care unit: a randomized clinical trial. Br J Anaesth. 2010;104:643–7.
- Robinson JD, Lupkiewicz SM, Palenik L, Lopez LM, Ariet M. Determination of ideal body weight for drug dosage calculations. Am J Hosp Pharm. 1983;40:1016–19.
- O'Driscoll BR, Howard LS, Davison AG. BTS guideline for emergency oxygen use in adult patients. Thorax. 2008;63:vi1–68.
- Lundquist H, Hedenstierna G, Strandberg A, Tokics L, Brismar B. CT-assessment of dependent lung densities in man during general anaesthesia. Acta Radiol. 1995;36:626–32.
- Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27.
- Neumann P, Rothen HU, Berglund JE, Valtysson J, Magnusson A, Hedenstierna G. Positive end-expiratory pressure prevents atelectasis during general anaesthesia even in the presence of a high inspired oxygen concentration. Acta Anaesthesiol Scand. 1999;43:295–301.
- Maisch S, Reissmann H, Fuellekrug B, Weismann D, Rutkowski T, Tusman G, et al. Compliance and dead space fraction indicate an optimal level of positive end-expiratory pressure after recruitment in anesthetized patients. Anesth Analg. 2008;106:175–81.
- Eichenberger A, Proietti S, Wicky S, Frascarolo P, Suter M, Spahn DR, et al. Morbid obesity and postoperative pulmonary atelectasis: an underestimated problem. Anesth Analg. 2002;95:1788–92.
- Strandberg A, Tokics L, Brismar B, Lundquist H, Hedenstierna G. Constitutional factors promoting development of atelectasis during anaesthesia. Acta Anaesthesiol Scand. 1987;31:21–4.
- Milic-Emili J, Torchio R, D'Angelo E. Closing volume: a reappraisal (1967-2007). Eur J Appl Physiol. 2007;99:567–83.
- Kang H, Park HJ, Baek SK, Choi J, Park SJ. Effects of preoxygenation with the three minutes tidal volume breathing technique in the elderly. Korean J Anesthesiol. 2010;58:369–73.
- McCarthy G, Elliott P, Mirakhur RK, McLoughlin C. A comparison of different pre-oxygenation techniques in the elderly. Anaesthesia. 1991;46:824–7.
- Edmark L, Auner U, Lindbäck J, Enlund M, Hedenstierna G. Postoperative atelectasis - a randomised trial investigating CPAP/PEEP during anaesthesia and FIO2 0.3 before extubation. Acta Anaesthesiol Scand. 2014; forthcoming; Doi: 10.1111/aas.12322.
- Benatar SR, Hewlett AM, Nunn JF. The use of iso-shunt lines for control of oxygen therapy. Br J Anaesth. 1973;45:711–18.
- Zetterström H. A slide-rule for assessment of venous admixture. Acta Anaesthesiol Scand. 1989;33:250–4.
- Tanoubi I, Drolet P, Donati F. Optimizing preoxygenation in adults. Can J Anaesth. 2009;56:449–66.
- Edmark L, Auner U, Enlund M, Östberg E, Hedenstierna G. Oxygen concentration and characteristics of progressive atelectasis formation during anaesthesia. Acta Anaesthesiol Scand. 2011;55:75–81.
- Licker MJ, Aldenkortt M. Perioperative lung protective strategies. J Pulmonar Respirat Med. 2012;S2:002– doi: 10.4172/2161-105X.S2-002.
- Aldenkortt M, Lysakowski C, Elia N, Brochard L, Tramer MR. Ventilation strategies in obese patients undergoing surgery: a quantitative systematic review and meta-analysis. Br J Anaesth. 2012;109:493–502.