Abstract
Background. The aim of this paper was to describe and analyze the effect of antibiotic policy changes on antibiotic consumption in Swedish hospitals and to review antibiotic stewardship in Swedish hospitals.
Results. The main findings were: 1) Antibiotic consumption has significantly increased in Swedish hospitals over the last decade. The consumption of cephalosporins has decreased, whereas that of most other drugs including piperacillin-tazobactam, carbapenems, and penicillinase-sensitive and -resistant penicillins has increased and replaced cephalosporins. 2) Invasive infections caused by ESBL-producing Escherichia coli and Klebsiella pneumoniae have increased, but the proportion of pathogens resistant to third-generation cephalosporins causing invasive infections is still very low in a European and international perspective. Furthermore, the following gaps in knowledge were identified: 1) lack of national, regional, and local data on the incidence of antibiotic resistance among bacteria causing hospital-acquired infections e.g. bloodstream infections and hospital-acquired pneumonia—data on which standard treatment guidelines should be based; 2) lack of data on the incidence of Clostridium difficile infections and the effect of change of antibiotic policies on the incidence of C. difficile infections and infections caused by antibiotic-resistant pathogens; and 3) lack of prospective surveillance programs regarding appropriate antibiotic treatment, including selection of optimal antimicrobial drug regimens, dosage, duration of therapy, and adverse ecological effects such as increases in C. difficile infections and emergence of antibiotic-resistant pathogens.
Conclusions. Evidence-based actions to improve antibiotic use and to slow down the problem of antibiotic resistance need to be strengthened. The effect of such actions should be analyzed, and standard treatment guidelines should be continuously updated at national, regional, and local levels.
Background
Interventions to restrict and improve antibiotic use may slow down the problem of resistance (Citation1) and are urgently needed in health care settings throughout the world. Antibiotic resistance causes increased mortality and morbidity, and higher costs (Citation2). In the European Union an estimated 25,000 deaths occur each year secondary to multidrug-resistant infections (Citation3). In low-income countries outside Europe there are no reliable estimates of the human burden of antibiotic resistance, but it is almost certainly higher than in Europe. The cost of treating a patient suffering a severe infection caused by a multidrug-resistant organism will by far exceed the cost of treating a patient infected by a susceptible organism of the same species. This is partly caused by a longer disease duration due to failure of initial treatment, and partly the need for hospitalization and more expensive drugs (Citation2). The World Health Organization and the European Commission have launched action plans to combat antimicrobial resistance (Citation4,5). A systematic program for rational use of antibiotics in hospitals, usually termed ‘antimicrobial stewardship', should include monitoring of appropriate use of antibiotics, and interventions to improve this by promoting an optimal antimicrobial drug regimen including agent, dose, duration of therapy, and route of administration (Citation6). The overall aim of antibiotic stewardship in hospitals is to optimize antibiotic use and clinical outcome so as to limit the emergence of antimicrobial-resistant bacteria and adverse events including antibiotic-associated diarrhea. Antibiotic stewardship should always be combined with actions to improve infection control and thereby reduce health care-associated infections. In most countries there is no legislation forcing health care providers to optimize their use of antimicrobial therapy. In a recently published Cochrane review and meta-analysis, the authors showed that interventions to reduce excessive antibiotic prescribing to patients in hospitals can reduce antimicrobial resistance or hospital-acquired infections, and interventions to increase effective prescribing can improve clinical outcome (Citation1). This meta-analysis supports the use of restrictive interventions when the need is urgent but suggests that persuasive and restrictive interventions are equally effective after six months. The Swedish Strama network (Collaboration against Antibiotic Resistance) has initiated interventions preventing the over-use of antibiotics in primary health care, including campaigns aimed at physicians, patients, and the media. These actions have been successful in reducing the Swedish outpatient antibiotic consumption measured as prescriptions per 1,000 inhabitants and year (Citation7). Approximately 10% of the total prescription of antibiotics in humans in Sweden occurs in hospitals. The aim of this paper was to analyze the effect of policy changes on antibiotic consumption in Swedish hospitals, and to review antibiotic stewardship in Swedish hospitals.
Interventions in antibiotic policy in Swedish hospitals
The Strama network, together with local drug and therapeutic committees, promoted the following changes in antibiotic policy in Swedish hospitals: 1) moderately severe (CRB-65 0-1) community-acquired pneumonia (CAP) should be treated with narrow-spectrum penicillins; 2) surgical prophylaxis should normally be given as one dose except in high-risk situations where 24 h is a maximum with few exceptions; 3) uncomplicated lower urinary tract infections in women should be treated with pivmecillinam or nitrofurantoin, including hospital inpatients, whereas the use of fluoroquinolones should be restricted; 4) extended-spectrum cephalosporins and fluoroquinolones should not be used in situations where treatment with a narrow-spectrum penicillin is an alternative.
Antibiotic consumption in Swedish hospitals, measured as prescribed doses in point prevalence studies
One in every three patients in Swedish hospitals received an antibiotic, according to Strama's repeated point prevalence studies (PPS) performed in 2003, 2004, 2006, 2008, and 2010 (Citation8). One patient in five was treated for a community-acquired infection, 1 in 10 for a health care-associated infection, and 1 in 15 received perioperative antibiotic prophylaxis (Citation8).
The point prevalence studies showed a 9% increase in the total number of patients treated with any antibiotic from 2003 to 2010. During the same period there was an 18% decrease in hospital prescription of cephalosporins, and a decrease in the prescription of fluoroquinolones, as well as a trend towards shorter surgical prophylaxis, indicating adherence to imposed policy changes. Cephalosporins were in particular replaced by piperacillin-tazobactam, and to some extent by narrow-spectrum penicillins and carbapenems, explaining the overall increased use of antibiotics in hospitals as revealed by these point prevalence studies. The studies comprised 24,655 patients, but as yet we cannot confirm that these changes in prescription were statistically significant (Citation8). Surveillance showing the percentage of appropriate choice of antimicrobial drug regimen, dose, duration of therapy, and route of administration is warranted if we are to determine whether or not further policy changes are required.
Antibiotic consumption in Swedish hospitals and nursing homes, based on sales statistics
Antimicrobial use in hospital care was measured as defined daily dosages (DDD)/thousand inhabitants and day (TIND). The number of DDDs was obtained from Apotekens Service AB (Citation7) and the Swedish eHealth Agency. These hospital care data are from all Swedish hospitals as well as from those nursing homes and other care providers that order antibiotics via requisition. Based on these data a statistical trend analysis was performed using linear regression, and a P value < 0.05 was considered statistically significant.
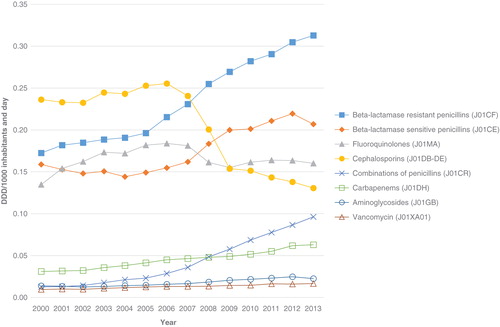
Overall antibiotic hospital consumption increased from 1.18 DDD/TIND in the year 2000 to 1.63 DDD/TIND in 2012 (+38%) (P < 0.001). The corresponding figures for DDD/TIND were as follows: for cephalosporins 0.24 to 0.14 (–71%) (P < 0.001); isoxazolyl-penicillins 0.17 to 0.30 (+76%) (P < 0.001); beta-lactamase-sensitive penicillins (PcG and PcV) 0.16 to 0.22 (+38%) (P < 0.001); fluoroquinolones 0.13 to 0.16 (+23%) (P = 0.46); piperacillin-tazobactam and amoxicillin-clavulanic acid 0.01 to 0.09 (+900%) (P < 0.001); carbapenems 0.03 to 0.06 (+100%) (P < 0.001); aminoglycosides 0.01 to 0.02 (+100%) (P < 0.001); and for vancomycin 0.01 to 0.02 (+100%) (P < 0.001) (). Corresponding significant changes were seen from 2000 to 2013, but data were missing for Jönköping county in November–December 2013.
Methodological problems with antibiotic sales statistics
The use of antibiotic sales statistics to measure the effects of antibiotic policy changes has the advantage of easy access but has several limitations since it does not show the true exposure, i.e. the prescribed daily dose (PDD) which is demonstrated in point prevalence studies (Citation8). In Sweden, the previously recommended standard doses of cefuroxime (1.5 g × 3 = 4.5 g/d) and cefotaxime (1 g × 3 = 3 g/d) do not conform to the WHO definition of DDD; 3 g/d and 4 g/d, respectively. According to Strama's repeated point prevalence studies (PPS) were the mean PDDs for cefuroxime and cefotaxime 3.5 and 3.1 g/d respectively, thus differed from DDDs as defined by WHO.
During the 1990s cefotaxime replaced cefuroxime in most Swedish hospitals after the recommendation from the Swedish Reference Group for Antibiotics (SRGA) (Citation9) to abandon the use of cefuroxime in the treatment of Gram-negative infections, due to its inferior performance compared with cefotaxime (Citation10). The fall in sales of cephalosporins may thus be overestimated as a result of this policy change since DDDs do not correspond to the commonly used dosages in Sweden. On the other hand, it is now recommended that the dose of beta-lactams for severe sepsis and septic shock be increased (Citation11). As a response to this some counties now use cefotaxime 2 g three times daily (Citation12), which leads to an overestimation of numbers of patients treated with cefotaxime as measured by DDDs. In a similar way, the WHO DDD for PcG is 3.6 g, but according to previous PPS data (all studies, n = 754 (Citation7)) the average prescribed daily dose for adults was 6.9 g, and thus DDDs based on sales statistics overestimate the use of narrow-spectrum penicillins.
Antibiotic consumption in hospitals, measured as DDD
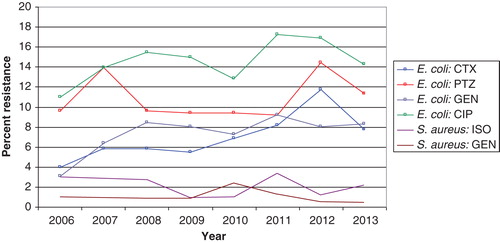
The most recent data available concerning Swedish hospital sales statistics with only hospital consumption included (nursing homes and other care providers excluded) are from 2012 and show a wide variation between counties in the use of narrow-spectrum penicillins, ranging from 6% to 21% of the total hospital consumption measured as DDDs (Citation7). Cephalosporin use varied between 4% and 15%, and the corresponding figures for fluoroquinolones were 9% to 14%, 4% to 8% for piperacillin-tazobactam, and 3% to 8% for carbapenems (Citation7). This four-fold difference, between counties in cephalosporin consumption, based on sales statistics and measured in DDDs, may have several explanations. It may also be a true difference in prescribed doses due to a difference in local standard treatment regimens. It may be an artifact due to the higher standard doses for cefotaxime (cefotaxime 2 g × 3 instead of 1 g × 3) in some counties. Furthermore, higher rates of non-susceptibility to piperacillin-tazobactam compared with cephalosporins have been reported among Escherichia coli in some hospitals including Östergötland (11.5% versus 3.8% in 2007, personal communication Lennart E. Nilsson, Linköping University) and Karolinska University Hospital, Stockholm (14% versus 6% in 2007) (). This has led to cefotaxime being the more rational empirical choice for E. coli sepsis in these settings, which could explain the relatively high consumption of third-generation cephalosporins. However, half of the proportion of E. coli with non-susceptibility to piperacillin-tazobactam was in the intermediate category and may be treated with piperacillin-tazobactam 4 g × 4, provided there is an adequate source control (Citation13). In future presentations of antibiotic consumption in hospitals we recommend comparison of consumption in primary, secondary, and tertiary care hospitals within each county since the mix of patient cases may vary with the level of hospital specialization (more severe infections at the tertiary level).
Total antibiotic consumption in hospitals, measured as DDD/100 patient-days
These data are based on sales statistics where data on the day of admission are provided as the denominator. The total antibiotic consumption in hospitals (nursing homes and other care providers excluded), measured as DDD/100 patient-days, increased by 9% between 2009 and 2012 (Citation7). Correspondingly, the consumption of cephalosporins decreased, being replaced by piperacillin-tazobactam/amoxicillin with or without clavulanic acid, carbapenems, and penicillinase-sensitive and -resistant penicillins.
Summary and analysis of antibiotic use in Swedish hospitals
Based on sales statistics the overall antibiotic consumption in hospitals and nursing homes and other settings that order antibiotics via requisition increased by 38% between 2000 and 2012 (P < 0.001) (see above). Measured as DDD/100 patient-days, hospital antibiotic use increased by 9% between 2009 and 2012 (Citation7). The point prevalence studies showed a 9% increase, between 2003 and 2010, of the total number of patients treated with any antibiotic. All data sources showed a reduction in the prescription of cephalosporins being replaced by piperacillin-tazobactam, carbapenems, as well as penicillinase-sensitive and -resistant penicillins. There were large differences between counties in use of different antibiotic groups. No analysis has been performed regarding the appropriateness of treatment, but this type of analysis may be carried out in point prevalence studies.
Use of PDDs is recommended in future analyses of the impact of antibiotic policy interventions on antibiotic use, since it measures what the patient actually receives and there are fewer flaws in data. PDD data can usually be collected from the hospital computer prescription systems or manually in point prevalence studies where the precision of antibiotic prescriptions can be assessed.
ESBL-producing Enterobacteriaceae
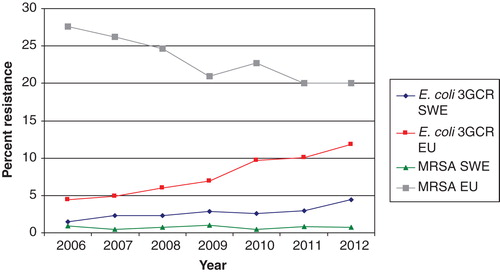
The incidence of extended spectrum betalactamase (ESBL)-producing E. coli (reported as cases per 100,000 inhabitants) has increased rapidly in all Swedish counties but is still low in the European perspective () (Citation7). The increasing incidence of ESBL-producing E. coli (ESBL-EC) bloodstream infections, a largely community-acquired or endogenous infection, is mainly influenced by factors other than hospital antibiotic consumption, international travel probably being the major cause of the rapid increase in ESBL-EC in Sweden (Citation14). The fall in hospital consumption of cephalosporins may lower selective pressure of these strains and prevent spread of highly transmissible strains, in particular ESBL-producing Klebsiella pneumoniae, in the hospital setting (Citation15). In the UK a successful antibiotic policy change has been achieved, lowering cephalosporin and fluoroquinolone consumption and reducing the incidence of Clostridium difficile infections (Citation16). However, there has been a substantial increase in use of amoxicillin-clavulanic acid, carbapenems, and piperacillin-tazobactam (Citation16). Livermore et al. reported a relative decrease in cephalosporin and fluoroquinolone resistance among E. coli in bloodstream infections after the above-mentioned change in antibiotic use, but the total number of bloodstream infections caused by non-susceptible E. coli increased (Citation17). Thus, there were 12% more cephalosporin-resistant cases of E. coli bacteremia in 2011 than in 2007, although the percentage resistance rates were higher in the first years (Citation17). Furthermore, according to the most recent data from EARS-Net, the number of third-generation cephalosporin resistant E. coli and K. pneumoniae in the UK returned to an all-time high in 2012 (Citation18). The replacement of cephalosporins with piperacillin-tazobactam and carbapenems has also been the case in Sweden (Citation7). It is not possible to determine on a national level, neither in the UK nor Sweden, if replacement of cephalosporins with other antibiotics has led to a slowing down of the rise in invasive isolates of ESBL-producing E. coli and K. pneumoniae. However, reduction in the use of cephalosporins has been shown to be successful in ESBL-producing K. pneumoniae outbreaks (Citation15). In future surveillance programs we recommend monitoring changes in the hospital prescription of antibiotics and incidence of antibiotic-resistant bacteria and C. difficile at hospital and ward levels, since this may vary widely. For this reason interventions have to be adapted taking this into account, and evaluation must also take place at the local level.
Clostridium difficile
C. difficile infection is a frequently encountered health care-associated infection in hospitals. In the Cochrane report on ‘Interventions to improve antibiotic prescribing practices for hospital inpatients' five studies were included on interventions against C. difficile infections (Citation1). All of the studies used the interrupted time series analysis method: one reported change in the intended direction by at least 15% and four by at least 50% (Citation1). Only one of five studies had reliable data on the effect of intervention of antibiotic prescribing, and this study showed the most pronounced effect on C. difficile infections. However, this was not statistically significant, probably because of too few observations prior to the study. A nationwide Swedish surveillance program for C. difficile infections was initiated by the Swedish Institute for Communicable Disease Control in 2009 (Citation7). The program included voluntary laboratory reporting of all new cases of C. difficile infection, plus determination of resistance and epidemiologic typing of isolates collected from clinical microbiology laboratories during two weeks every year (). In 2012, a total of 491 isolates were collected, and none of the isolates was resistant to the then available treatment options, metronidazole and vancomycin. Three other antibiotics were also tested (moxifloxacin, clindamycin, erythromycin), not because they represent any treatment alternative, but as a marker for selective pressure. Isolates with resistance to moxifloxacin, e.g. ribotypes 012 and 231, from 2009 to 2012 were clustered in geographical regions. This may have been caused by local outbreaks of resistant isolates or selection bias (Citation7). No considerable transmission of the highly virulent ribotype 027 has been observed in Sweden.
Figure 4. PCR ribotyping of C. difficile isolates collected from Swedish clinical microbiology laboratories during weeks 11 and 39, 2013. (Thomas Åkerlund, Department of Public Health, Sweden).
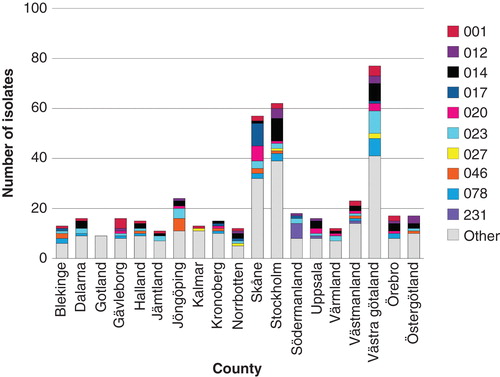
Six isolates of ribotype 027 were seen in 2013, but only two of these six isolates were moxifloxacin-resistant and there was no clustering (). The first outbreak of ribotype 027 was described in Växjö Hospital in the county of Kronoberg in December 2013 (Thomas Åkerlund, The Department of Public Health, personal communication). Infection control precautions and antibiotic policy interventions to prevent transmission of C. difficile between patients must be based on up-to-date knowledge (Citation19).To measure the effect of interventions against C. difficile, indicators need to be developed, such as hospital antibiotic consumption in DDDs/100 patient-days versus number C. difficile events/100 patient-days, cost of cleaning/100 admissions, and quality of cleaning including adherence to hygiene rules (Citation20).
Invasive bacterial infections
In Sweden the incidence of invasive infections caused by ESBL-producing E. coli is increasing but is still at a level of only one-third of the average level in Europe () (Citation7). In fact, the proportion of major community-acquired pathogens including E. coli, K. pneumoniae, and Staphylococcus aureus resistant to third-generation cephalosporins is still very small in the European and international perspective () (Citation2). Cefotaxime is still recommended as empirical treatment for severe sepsis by the Infectious Disease Society of Sweden, but many counties have successfully lowered cephalosporin use in order to avoid selection of ESBL-producing Enterobacteriaceae. Cefotaxime resistance among invasive isolates of E. coli increased from a low frequency of 1.5% in 2006 to 4.4% in 2012, and cefotaxime resistance among K. pneumoniae isolates increased from 1.5% to 2.6% over the same period () (Citation7). These figures are based on pooled data from community- and hospital-acquired infections. No cefotaxime resistance has been reported among invasive isolates of Neisseria meningitidis (data not shown). The MRSA rate among invasive isolates remained stable and below 1% from 2006 through 2012 in Sweden () (Citation7). This indicates that empirical treatment of severe community-acquired sepsis with a third-generation cephalosporin is still appropriate since it covers 95%–100% of the major pathogens encountered in this situation. However, a more systematic step-down strategy should be implemented for Gram-positive pathogens, including change to narrow-spectrum penicillins (benzylpenicillin or cloxacillin), as soon as susceptibility data are available and the infectious focus is outside the central nervous system. The susceptibility to benzyl penicillin among invasive isolates of K. pneumoniae and N. meningitidis in 2012 was 95% and 83%, respectively (Citation7). Thus, benzyl penicillin is no longer an appropriate empirical treatment for N. meningitidis sepsis. The low risk of failure of a standard empirical treatment for sepsis in Sweden was confirmed in a recent study which showed that 93% of patients with severe sepsis or septic shock admitted to a Swedish ICU received adequate empirical antibiotic therapy (Citation21). There are no national data available on the susceptibility among invasive isolates of E. coli, K. pneumoniae, and Pseudomonas aeruginosa to the more and more frequently used piperacillin-tazobactam, but data comparing susceptibility to cefotaxime and piperacillin-tazobactam in invasive E. coli in the Karolinska University Hospital are provided in .
According to a recent multi-center study that included Swedish hospitals, an increase in the incidence of nosocomial bloodstream infections due to antibiotic-resistant bacteria has occurred in addition to infections caused by antibiotic-susceptible bacteria, increasing the total burden of nosocomial bloodstream infections (Citation22). However, since only local hospital data are available for patients in Sweden there is no nationwide picture of the problem of nosocomial bloodstream infections caused by antibiotic-resistant bacteria.
Antibiotic guidelines for patients suffering from nosocomial infections should be based on the local microbiologic surveillance results of each department and related to underlying diseases and risk of infection with pathogens difficult to treat such as Pseudomonas or Candida resistant to standard treatment (Citation23).
Conclusions
The main findings of this study were: 1) Antibiotic consumption has increased in Swedish hospitals over the last decade. The consumption of cephalosporins has decreased, whereas that of most other drugs including piperacillin-tazobactam, carbapenems, and penicillinase-sensitive and -resistant penicillins has increased, thus partly replacing cephalosporins. 2) Invasive infections caused by ESBL-producing E. coli and K. pneumoniae have increased, but the proportion of pathogens resistant to third-generation cephalosporins causing invasive infections is still very low in the European and international perspective. 3) We have identified the following gaps in knowledge: 1) lack of national, regional, and local data on the incidence of antibiotic resistance among bacteria causing hospital-acquired bloodstream infections and hospital-acquired pneumonia, data on which standard treatment guidelines should be based; 2) lack of a program for the surveillance of C. difficile infections, and for assessment of the effects of changes in antibiotic use on the incidence of C. difficile infections and infections caused by antibiotic-resistant pathogens; 3) lack of prospective surveillance programs regarding appropriate antibiotic treatment, including selection of optimal antimicrobial drug regimens, dosage, duration of therapy, and adverse ecological effects such as increases in C. difficile infections and emergence of antibiotic-resistant pathogens. Evidence-based actions to improve antibiotic use and a retardation of the resistance problem must be strengthened. The effect of these actions should be assessed, and standard treatment guidelines should be continuously up-dated at national, regional, and local levels.
Acknowledgements
Our thanks go to Thomas Åkerlund and Barbro Olsson Liljequist at The Public Health Agency of Sweden, and to all laboratories in Sweden for providing C. difficile typing and susceptibility data (EARS-Net).
Declaration of interest: The authors report no conflicts of interest. Christian G. Giske has received speaker's honorarium from AstraZeneca and Meda. The authors alone are responsible for the content and writing of the paper.
References
- Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;4:CD003543.
- de Kraker ME, Davey PG, Grundmann H; BURDEN Study Group. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011;8:e1001104.
- European Centre for Disease Prevention and Control. The bacterial challenge: time to react. A call to narrow the gap between multidrug-resistant bacteria in the EU and the development of new antibacterial agents. Stockholm: European Centre for Disease Prevention and Control, 2009. Available at http://www.ecdc.europa.eu/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf. accessed 14 January 2014.
- Leung E, Weil DE, Raviglione M, Nakatani H; World Health Organization World Health Day Antimicrobial Resistance Technical Working Group. The WHO policy package to combat antimicrobial resistance. Bull World Health Organ. 2011;89:390–2.
- European Commission. The European Commission's 2011 action plan against the rising threats from antimicrobial resistance. 2011. Available at http://ec.europa.eu/dgs/health_consumer/docs/communication_amr_2011_748_en.pdf. accessed 14 January 2014.
- Infectious Diseases Society of America. Promoting Antimicrobial Stewardship in Human Medicine. Available at http://www.idsociety.org/stewardship_policy/#sthash.xKOIwFmV.dpuf. accessed 14 January 2014.
- Swedish Institute for Communicable Disease Control, National Veterinary Institute. A Report on Swedish Antibiotic Utilisation and Resistance in Human Medicine (SWEDRES) and Swedish Veterinary Antimicrobial Resistance Monitoring (SVARM). 2012. Available at http://www.folkhalsomyndigheten.se/publicerat-material/publikationer/SWEDRESSVARM-2012/. accessed 14 January 2014.
- Strama. Swedish point prevalence studies on antibiotic use in hospitals. 2003-2010. Available at http://folkhalsomyndigheten.se/amnesomraden/statistik-och-undersokningar/antibiotikastatistik/sverige/slutenvard/. accessed 17 March 2014.
- Hanberger H, Odenholt I, Giske CG, Kahlmeter G. [Time to abandon “the house wine”. Stop for uncritical empiric use of cefuroxime]. Lakartidningen. 2009;106:291–2.
- Dornbusch K, Bengtsson S, Brorson JE, Fritz H, Henning C, Kronvall G, et al. Susceptibility to beta-lactam antibiotics and gentamycin of gram-negative bacilli isolated from hospitalized patients: a Swedish multicenter study. Scand J Inf Dis. 1988;20:641–7.
- Taccone FS, Laterre PF, Dugernier T, Spapen H, Delattre I, Wittebole X, et al. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care. 2010;14:R126.
- Swedish Society of Infectious Diseases, Sepsis Working Group. Guidelines for severe sepsis and septic shock. 2012. Available at http://www.infektion.net/v%C3%A5rdprogram-f%C3%B6r-sv%C3%A5r-sepsisseptisk-chock. accessed 14 January 2014.
- EUCAST. Piperacillin-tazobactam, Rationale for the EUCAST clinical breakpoints, version 1.0. 2010. Available at http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Piperacillin-tazobactam_rationale_ Nov2010_v_1.0.pdf. accessed 20 March 2014.
- Ostholm-Balkhed A, Tarnberg M, Nilsson M, Nilsson LE, Hanberger H, Hallgren A, et al. Travel-associated faecal colonization with ESBL-producing Enterobacteriaceae: incidence and risk factors. J Antimicrob Chemother. 2013;68:2144–53.
- Tangden T, Eriksson BM, Melhus A, Svennblad B, Cars O. Radical reduction of cephalosporin use at a tertiary hospital after educational antibiotic intervention during an outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2011;66:1161–7.
- Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J; ARHAI Antimicrobial Stewardship Group. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart--Then Focus. J Antimicrob Chemother. 2012;67 (Suppl 1):i51–63.
- Livermore DM, Hope R, Reynolds R, Blackburn R, Johnson AP, Woodford N. Declining cephalosporin and fluoroquinolone non-susceptibility among bloodstream Enterobacteriaceae from the UK: links to prescribing change? J Antimicrob Chemother. 2013;68:2667–74.
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial resistance interactive database (EARS-Net). 2013. Available at http://www.ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/database/Pages/table_reports.aspx. accessed 16 March 2014.
- Otete EH, Ahankari AS, Jones H, Bolton KJ, Jordan CW, Boswell TC, et al. Parameters for the mathematical modelling of Clostridium difficile acquisition and transmission: a systematic review. PLoS One. 2013;8:e84224.
- Toepfer M, Magnusson C, Noren T, Hansen I, Iveroth P, Offenbartl K. [Insidious and widespread outbreak of Clostridium difficile. Changed cleaning procedures and frequent evaluations cut infection rates in half]. Lakartidningen. 2014;111:24–7.
- Linner A, Sunden-Cullberg J, Johansson L, Hjelmqvist H, Norrby-Teglund A, Treutiger CJ. Short- and long-term mortality in severe sepsis/septic shock in a setting with low antibiotic resistance: a prospective observational study in a Swedish university hospital. Front Public Health. 2013;1:51.
- Ammerlaan HS, Harbarth S, Buiting AG, Crook DW, Fitzpatrick F, Hanberger H, et al. Secular trends in nosocomial bloodstream infections: antibiotic-resistant bacteria increase the total burden of infection. Clin Infect Dis. 2013;56:798–805.
- Pittet D, Li N, Woolson RF, Wenzel RP. Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin Infect Dis. 1997;24:1068–78.