Abstract
We present the case of a 27-year-old female with subcortical osteonecrosis of the humeral capitulum. Percutaneous retrograde drilling of the lesion and application of recombinant human bone morphogenetic protein (BMP)-7 were combined with autologous bone grafting. At follow-up the patient was almost pain-free, had normalized her range of motion, and radiography showed consolidation of the lesion without any heterotopic bone formation. By timing surgery prior to subchondral collapse, biomechanical stability of the subchondral bone was maintained. To our knowledge, this is the first report on the treatment of an osteonecrosis in this location with a BMP, and this strategy could potentially be applied in other locations with juxta-articular osteonecrosis.
Introduction
Osteochondritis dissecans of the capitulum humeri is a relatively rare elbow condition. Mostly adolescents or young adults are affected, and a higher incidence of the disease has been reported in baseball players and gymnasts (Citation1). In analogy to other osteonecrotic lesions the disease is characterized by a temporal pattern, with a final stage of compensatory repair that either results in joint congruity or incongruity and secondary osteoarthritis (Citation2). The lesion is typically located in the anterolateral aspect of the capitulum. Left untreated, about half of the patients with osteochondritis dissecans of the capitulum humeri develop osteoarthritis of the elbow (Citation3,4). Treatment is usually conservative in early stages prior to fragment dissection, whereas end-stage osteochondritis dissecans is frequently treated by extirpation of the free bodies (Citation5). However, the development of osteochondral or osteoperiosteal transplantation techniques for other joints affected by subchondral osteonecrosis rejuvenated interest in the surgical treatment of early osteochondritis dissecans of the capitulum humeri (Citation6–8).
The molecular mechanisms underlying subchondral osteonecrosis and subsequent remodeling are now better understood, and the pivotal role of bone morphogenetic proteins (BMP) in the process of bone regeneration has been recognized (Citation9). Different preparations of BMPs, primarily BMP-2 and BMP-7, are in clinical use in order to enhance bone healing (Citation10–12). BMPs have been sporadically used in order to enhance bone regeneration in osteochondritis dissecans, but never in the elbow (Citation13,14).
Case report
A 27-year-old female was referred to our unit because of pain in the right elbow. The patient located her pain to the radial side of the joint, and exacerbation occurred after carrying heavy objects and manual work. Her present medical history was characterized by Crohn’s disease that had previously been treated with prednisolone (50 mg/day), and on admission she received a tumor necrosis factor (TNF)-α antagonist on a weekly basis (Certolizumab pegol, Cimzia®, UCB, Brussels, Belgium).
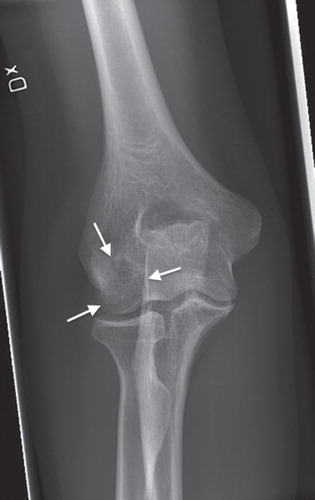
Upon clinical examination a slight swelling and tenderness over the radial humeral epicondyle and the capitulum humeri were noted. Full extension and a flexion lag of 15 degrees were found compared to the contralateral side. Pronation was free, whereas supination was painful and reduced. Plain anteroposterior and lateral radiography showed a localized radiolucency typical of osteonecrosis in the capitulum humeri, but without indication of subchondral fracture or fragment dissection (). MRI confirmed the diagnosis of osteochondritis dissecans and also indicated that the articular cartilage had not yet collapsed.
Figure 1. Standard preoperative anteroposterior radiography of the elbow showing the osteonecrotic lesion in the capitulum humeri.
At surgery a small incision was made over the dorsal aspect of the radial epicondyle. Using fluoroscopy a retrograde drilling of the osteonecrosis was performed using a guide-wire followed by a 3.5 mm cannulated drill. Subsequently, a cancellous bone graft measuring 0.5 cm3 was obtained from the right iliac crest and mixed with one dose of BMP-7 (Osigraft®, Stryker, Montreux, Switzerland) according to the manufacturer’s instructions. This mixture of cancellous bone and BMP-7 was then inserted through the drill hole up to the subcortical bone by use of a blunt trocar. The periosteum was then meticulously closed with resorbable sutures and the wound thoroughly washed prior to closure. Postoperatively the patient was allowed unrestricted mobilization, although handling of weights above 1 kg was prohibited during the first 2 months. Wound healing was unremarkable at both the recipient and the donor site.
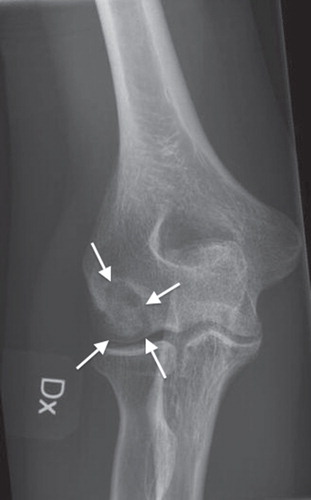
At follow-up 4 months postoperatively the patient described that she had improved with respect to daily activities and that she was now almost pain-free. Clinically, supination was no longer painful and the range of motion was improved, although a minimal flexion lag of 5 degrees persisted. Plain radiography showed that the previously osteonecrotic portion of the capitulum humeri had consolidated and no fragmentation or dissection had occurred (). Importantly, no signs of extraosseous calcification could be detected around the area of the surgical approach or in the joint.
Discussion
In this case of osteochondritis dissecans of the capitulum humeri we achieved a successful outcome in the short term by combining autografted bone with BMP (Citation6,13–15). This concept is based on the biological prerequisites that are also discussed in the context of fracture healing: potent osteogenic cell populations, osteoinductive stimuli, and osteoconductive matrix scaffolds (Citation16,17).
We optioned to use an autologous iliac crest bone graft, although other studies advocate usage of allografts to avoid donor site morbidity (Citation6). The choice of an autograft in our case was based on the presumed need for intact osteogenic mesenchymal stem cells and different growth factors known to enhance bone regeneration (Citation18). In order to optimize the osteoinductive stimulus we enriched our bone graft with BMP. This choice of treatment was based on previous reports describing that BMP itself may not have the chemotactic properties needed to attract mesenchymal stem cells to the site of injury, but only the ability to induce osteogenic differentiation (Citation13,14,19). In this case we used Osigraft® (BMP-7) due to its handling properties: BMP-2 (InductOs®) is applied to the site of bone healing dissolved in an absorbable type I collagen sponge carrier, whereas Osigraft® is mixed with a sodium chloride solution to make up a suspension with a consistency of wet sand. This latter solution was well suited for mixing with a cancellous bone graft and insertion through a small drill hole.
It is important to note that biomechanical prerequisites such as primary stability are necessary to obtain successful bone regeneration (Citation17). In osteonecrosis there is no obvious interfragmentary strain, but the bony structure surrounding the osteonecrosis site is covered by a brittle osteochondral fragment which is exposed to compression forces. Eventually, in the natural course of juxta-articular osteonecrosis, these forces result in fragmentation and dissection. This pathophysiology may explain why outcomes are poor after treatment of later stages of osteonecrosis in large joints with great compression forces. In such situations, the use of similar surgical procedures, osteogenic cell populations, osteoinductive stimuli, and osteoconductive matrix scaffolds may not be sufficient to induce bone healing (Citation14).
Conclusion
Osteonecrotic lesions of the capitulum humeri are difficult to treat and associated with a high degree of long-term disability. Here we present a successful treatment option based on a BMP-7-enriched autologous bone graft. This treatment strategy could potentially be applied to juxta-articular osteonecrosis in other non-weight-bearing joints.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Schenck RC Jr, Goodnight JM. Osteochondritis dissecans. J Bone Joint Surg Am. 1996;78:439–56.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteochondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216:207–12.
- Bauer M, Jonsson K, Josefsson PO, Linden B. Osteochondritis dissecans of the elbow. A long-term follow-up study. Clin Orthop Relat Res. 1992;284:156–60.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363:108–15.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89:1205–14.
- Mont MA, Etienne G, Ragland PS. Outcome of nonvascularized bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2003;417:84–92.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34:714–20.
- Kakinoki R, Ikeguchi R, Nakayama K, Yamakawa T, Morimoto Y, Nakamura T. Treatment of avascular necrosis of the capitulum of the humerus using a free vascularized osteoperiosteal graft from the medial condyle of the femur: a case report. J Shoulder Elbow Surg. 2008;17:e1–4.
- Marsell R, Einhorn TA. The role of endogenous bone morphogenetic proteins in normal skeletal repair. Injury. 2009;40:S4–7.
- Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–34.
- Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A:S151–8.
- Marsell R, Einhorn TA. Emerging bone healing therapies. J Orthop Trauma. 2010;24:S4–8.
- Seyler TM, Marker DR, Ulrich SD, Fatscher T, Mont MA. Nonvascularized bone grafting defers joint arthroplasty in hip osteonecrosis. Clin Orthop Relat Res. 2008;466:1125–32.
- Lieberman JR, Conduah A, Urist MR. Treatment of osteonecrosis of the femoral head with core decompression and human bone morphogenetic protein. Clin Orthop Relat Res. 2004;429:139–45.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90:47–62.
- Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–5.
- Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38:S3–6.
- Pape HC, Evans A, Kobbe P. Autologous bone graft: properties and techniques. J Orthop Trauma. 2010;24:S36–40.
- Bais MV, Wigner N, Young M, Toholka R, Graves DT, Morgan EF, et al. BMP2 is essential for post natal osteogenesis but not for recruitment of osteogenic stem cells. Bone. 2009;45:254–66.