Abstract
Background. The disease progression of patients with primary biliary cirrhosis (PBC) varies significantly, and the prognostic markers that identify those patients who will develop liver failure have been scarcely studied from a Chinese cohort.
Aims. We aimed to determine the predictive factors of liver failure in patients with PBC.
Methods. Patients who were first diagnosed as PBC with hepatic compensation between January 2007 and December 2009 were enrolled in this cohort study.
Results. Altogether 398 patients were finally included. Of these patients, 80% were women, 98% had positive antimitochondrial antibodies, and 45% had positive antinuclear antibodies (ANA). To December 2012, a total of 38 patients developed liver failure. According to the outcome, patients who developed liver failure had had higher serum concentration of baseline total bilirubin (TBil) (p = 0.013) and total bile acid (TBA) (p < 0.001), and lower concentrations of baseline total cholesterol (Tch) (p = 0.008), than patients who did not develop liver failure. Additionally, the proportion of ANA positivity was statistically different between the two groups (p = 0.009). In the established model for predicting liver failure in PBC, three variables were finally selected out, including Tch (odds ratio (OR) 0.552, 95% confidence interval (CI) 0.394–0.774, p < 0.001), TBA (OR 1.006, 95% CI 1.002–1.010, p = 0.002), and ANA (+ versus –, OR 5.518, 95% CI 1.155–26.376, p = 0.032).
Conclusions. ANA, Tch, and TBA are predictors of liver failure in PBC.
Introduction
Primary biliary cirrhosis (PBC) is an autoimmune liver disease characterized by the destruction of intrahepatic bile ducts, which can lead to hepatic cirrhosis and eventually liver failure and death (Citation1,2). Ursodeoxycholic acid (UDCA) is the only drug accepted internationally for the treatment of PBC (Citation3-5). However, a recent meta-analysis of 16 randomized clinical trials demonstrated no significant benefits of UDCA on all-cause mortality or liver transplantation in patients with PBC (Citation6). In fact, the disease progression varies markedly among patients with PBC (Citation7); moreover, substantial divergences of clinical characteristics exist because of variations in the populations under different studies (Citation8,9). Thus, it is necessary in the early stage to determine the prognostic variables associated with the development of end-stage liver disease, so that physicians can closely monitor the disease progress and adjust treatment measures in a timely manner before fatal events occur. In the present study, we aim to study the clinical characteristics and risk factors associated with the development of liver failure in a prospective cohort with PBC.
Patients and methods
Patients
Patients who were first diagnosed as PBC with hepatic compensation between January 2007 and December 2009 in Beijing 302 Hospital were enrolled in this cohort study. All of these included patients had received UDCA therapy at the initial diagnosis of PBC. Exclusion criteria included the concurrence of autoimmune hepatitis or extra-hepatic autoimmune diseases; infection with hepatitis A, B, C, D, E, Epstein–Barr virus, cytomegalovirus, or human immunodeficiency virus; the presence of other forms of liver diseases such as alcoholic liver disease, drug-induced hepatitis, or Wilson’s disease; the use of corticosteroids or immunosuppressive drugs for a period of more than 2 weeks; and UDCA non-responders. Liver failure in this study was defined as coagulopathy (prothrombin activity (PTA) ≤40% or international normalized ratio (INR) ≥1.5) and jaundice (serum total bilirubin (TBil) ≥171 μmol/L or a daily increase ≥17.1 μmol/L).
The study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of Beijing 302 Hospital.
Laboratory tests
Biochemical profiles, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), gamma glutamyl transferase (GGT), alkaline phosphatase (ALP), albumin, total cholesterol (Tch), and total bile acid (TBA) were measured using standard laboratory procedures. Normalized serum concentrations of ALT, AST, TBil, GGT, ALP, albumin, Tch, and TBA were, respectively, <40 U/L, <40 U/L, <17.1 μmol/L, 7–32 U/L, 40–150 U/L, 35–55 g/L, 2.8–5.2 mmol/L, and 0–10 μmol/L.
Serum autoantibodies, including antimitochondrial antibodies (AMA) and antinuclear antibodies (ANA), were tested using indirect immunofluorescence with standard methods (Euroimmun Medizinnische Labordiagnostika AG, Lubeck, Germany), and sera were considered to be positive when they produced a reaction at a dilution of ≥1:100. Immunoglobulin (Ig) was assayed by means of immunological turbidometry (Diasys Diagnostic Systems, Shanghai, China). Normal serum concentrations of IgA, IgG, and IgM were 0.69–3.28 g/L, 7.23–16.6 g/L, and 0.63–2.77 g/L, respectively.
Statistical analysis
Data analyses were performed using SAS 9.2 software (SAS Institute Inc., Cary, NC, USA). Continuous data were expressed as medians (interquartile range). Categorical data were expressed as the number of subjects. Group comparisons were performed using the Wilcoxon rank sum test for continuous variables, and chi-square test or Fisher exact test for categorical variables. Logistic regression was used for evaluating prognostic predictors of liver failure. A probability (p) value of less than 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
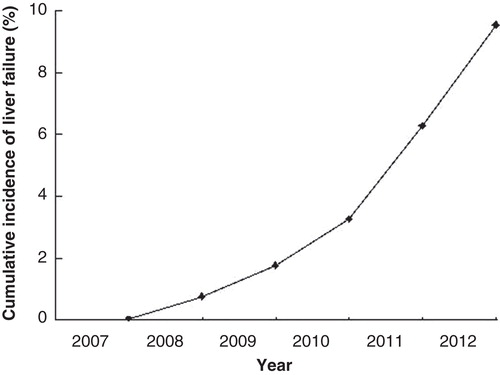
Finally, 398 patients were included. In these patients, 317 (80%) were female and 81 (20%) were male. The average age was 57 (12) years. A total of 389 patients had positive AMA, and 9 had negative AMA; 182 patients had positive ANA, and 216 had negative ANA. In the years 2007, 2008, and 2009, 80, 147, and 171 patients were enrolled (). To December 2012, a total of 38 patients had developed liver failure ().
Univariate analysis for baseline variables in PBC
According to the outcome, patients who developed liver failure had had higher serum concentrations of baseline TBil (43.7 μmol/L versus 23.2 μmol/L, p = 0.013) and TBA (92.5 μmol/L versus 46.0 μmol/L, p < 0.001) and lower serum concentrations of baseline Tch (3.09 mmol/L versus 4.52 mmol/L, p = 0.008) than patients who did not develop liver failure (). In addition, the proportion of ANA positivity differed between the two groups. Thus, a majority of the patients who developed liver failure had positive ANA (65.79% versus 43.61%, p = 0.009).
Table I. Comparison of baseline characteristics at entry between patients with and without liver failure.
Predictors of liver failure in PBC
Three variables were eventually selected out to predict the development of liver failure in PBC using logistic regression, including Tch (odds ratio (OR) 0.552, 95% confidence interval (CI) 0.394–0.774, p < 0.001), TBA (OR 1.006, 95% CI 1.002–1.010, p = 0.002), and ANA (+ versus –, OR 5.518, 95% CI 1.155–26.376, p = 0.032) ().
Table II. Predictors of liver failure in primary biliary cirrhosis.
Discussion
PBC is a chronic and progressive cholestatic disease, and its pathogenesis remains unclear (Citation10-12). Due to lack of curative therapeutics, liver failure is an evitable severe outcome in the majority of such patients. So, studying factors associated with the development of liver failure has vital and practical value. Beijing 302 Hospital is the largest hospital specializing in hepatology in China. Therefore, such an investigation on the risk of incipient liver failure in a large number of PBC patients possesses certain representativeness.
Previous studies have shown that patients with PBC often have higher serum concentrations of cholesterol (Citation13-15). Because the synthetic and metabolic process of cholesterol is closely associated with the function of the liver, the development and progression of liver diseases can influence the serum concentrations of cholesterol. In our study, patients with lower total serum cholesterol concentrations were more likely to develop liver failure than patients with higher concentrations.
TBA has been less studied than other serum markers in the evaluation of the prognosis of PBC. Based on our study, it was an independent risk factor for liver failure in PBC. As previously shown, cholestasis is a main physiopathological characteristic of PBC. It can cause the accumulation of hydrophobic bile acids in the liver, which are toxic to cellular membranes (Citation16,17). So, an increase of serum TBA may mirror the disease severity in PBC.
It has been reported that ANA can be found in 30%–50% of all PBC patients, and disease-specific ANA are associated with a more severe and rapidly progressing disease (Citation18-20). In the present study, ANA were detected in 46% of the patients and proved to be related to the occurrence of liver failure. Regarding other early variables, such as ALP, GGT, and IgM, no correlations with the development of liver failure were observed, though some of them had definitely diagnostic value.
In conclusion, ANA positivity, a lower serum concentration of Tch, and higher serum concentration of TBA are all associated with the development of liver failure in PBC.
Acknowledgements
We are grateful to Professor Yu-kun Han and Dr Xin-ying Liu for their great help in the patient enrollment. Zhen-man Wei was the guarantor; Pan Zhao designed the study; Wei-wei Liu, Jin-feng Li, and Pan Zhao analyzed the data; Hao-zhen Yang and Pan Zhao enrolled the patients; Pan Zhao, Hao Wang, Jun Xu, Rui-fang Wang, and Cheng Jin collected the data; Pan Zhao and Chun-ya Wang wrote the manuscript. All authors read and approved the final manuscript.
Pan Zhao, Wei-wei Liu, Jin-feng Li and Chun-ya Wang contributed equally to this work.
Declaration of interest: This work was partly supported by the 302 Hospital Research Project (YNKT2013009). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Czul F, Peyton A, Levy C. Primary biliary cirrhosis: therapeutic advances. Clin Liver Dis. 2013;17:229–42.
- Balmer ML, Dufour JF. Treatment of hypercholesterolemia in patients with primary biliary cirrhosis might be more beneficial than indicated. Swiss Med Wkly. 2008;138:415–19.
- Silveira MG, Brunt EM, Heathcote J, Gores GJ, Lindor KD, Mayo MJ. American Association for the Study of Liver Diseases endpoints conference: design and endpoints for clinical trials in primary biliary cirrhosis. Hepatology. 2010;52:349–59.
- Zhao P, Han Y. Low incidence of positive smooth muscle antibody and high incidence of isolated IgM elevation in Chinese patients with autoimmune hepatitis and primary biliary cirrhosis overlap syndrome: a retrospective study. BMC Gastrol. 2012;12:1.
- Uibo R, Kisand K, Yang CY, Gershwin ME. Primary biliary cirrhosis: a multi-faced interactive disease involving genetics, environment and the immune response. APMIS. 2012;120:857–71.
- Rudic JS, Poropat G, Krstic MN, Bjelakovic G, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev. 2012;12:CD000551.
- Ishibashi H, Komori A, Shimoda S, Ambrosini YM, Gershwin ME, Nakamura M. Risk factors and prediction of long-term outcome in primary biliary cirrhosis. Intern Med. 2011;50:1–10.
- Qin B, Liang Y, Yang Z, Zhong R. Scientific publications on primary biliary cirrhosis from 2000 through 2010: an 11-year survey of the literature. PLoS One. 2012;7:e35366.
- Karlsson-Parra A, Nyberg A, Tötterman TH, Lööf L, Forsum U. Primary biliary cirrhosis–phenotypic characterization of immunocompetent cells in peripheral blood and liver tissue. Ups J Med Sci. 1984;89:254–65.
- Zhao P, Yang HZ, Li JF, Wang CY, Liu XY, Zhong YW, et al. A case of autoimmune hepatitis and primary biliary cirrhosis overlap syndrome treated with Chinese herbs. Chin J Integr Med. 2013;19:468–70.
- Liberal R, Grant CR, Sakkas L, Bizzaro N, Bogdanos DP. Diagnostic and clinical significance of anti-centromere antibodies in primary biliary cirrhosis. Clin Res Hepatol Gastroenterol. 2013;37:572–85.
- Chong VH, Telisinghe PU, Jalihal A. Primary biliary cirrhosis in Brunei Darussalam. Hepatobiliary Pancreat Dis Int. 2010;9:622–8.
- Longo M, Crosignani A, Battezzati PM, Squarcia Giussani C, Invernizzi P, Zuin M, et al. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut. 2002;51:265–9.
- Alempijevic T, Sokic-Milutinovic A, Pavlovic Markovic A, Jesic-Vukicevic R, Milicic B, Macut D, et al. Assessment of metabolic syndrome in patients with primary biliary cirrhosis. Wien Klin Wochenschr. 2012;124:251–5.
- Cash WJ, O’Neill S, O’Donnell ME, McCance DR, Young IS, McEneny J, et al. Randomized controlled trial assessing the effect of simvastatin in primary biliary cirrhosis. Liver Int. 2013;33:1166–74.
- Dilger K, Hohenester S, Winkler-Budenhofer U, Bastiaansen BA, Schaap FG, Rust C, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133–40.
- Inamine T, Higa S, Noguchi F, Kondo S, Omagari K, Yatsuhashi H, et al. Association of genes involved in bile acid synthesis with the progression of primary biliary cirrhosis in Japanese patients. J Gastroenterol. 2013;48:1160–70.
- Shi TY, Zhang LN, Chen H, Wang L, Shen M, Zhang X, et al. Risk factors for hepatic decompensation in patients with primary biliary cirrhosis. World J Gastroenterol. 2013;19:1111–18.
- Gatselis NK, Zachou K, Norman GL, Gabeta S, Papamichalis P, Koukoulis GK, et al. Clinical significance of the fluctuation of primary biliary cirrhosis-related autoantibodies during the course of the disease. Autoimmunity. 2013;46:471–9.
- Bogdanos DP, Komorowski L. Disease-specific autoantibodies in primary biliary cirrhosis. Clin Chim Acta. 2011;412:502–12.